McOrist S, Bowles R, Blackall P. Autogenous sow vaccination for Glasser’s disease in weaner pigs in two large swine farm systems. J Swine Health Prod. 2009;17(2):90–96
| Case study | Peer reviewed |
Cite as: McOrist S, Bowles R, Blackall P. Autogenous sow vaccination for Glasser’s disease in weaner pigs in two large swine farm systems. J Swine Health Prod. 2009;17(2):90–96.
Also available as a PDF.
SummaryWe aimed to effectively vaccinate sows in two large farm systems suffering severe Glasser’s disease in their weaner pigs. System A or B supplied 4000 or 7000 weaned pigs weekly to their respective single-site nursery facilities. Suspect serosal lesions were cultured for Haemophilus parasuis, with serotyping and genotyping for tbpA and tbpB transferrin-binding genes. Most cultured H parasuis were either serotype 5 or 12 or nontypable. Specific tbpA and tbpB PCR products were further analyzed for restriction fragment length polymorphisms. This indicated that the dominant strains were genotypes bb and hg (Farm A), and aa, bb, and kf (Farm B). Selected dominant-strain cultures were expanded and formalin-killed for use as autogenous bacterins in sows 5 and 2 weeks prior to farrowing. Mortality and average daily weight gain in sheds housing progeny derived from vaccinated sows (mortality of 0.5% to 0.9% of total population) were lower than those in sheds housing nonvaccinated progeny (mortality of 1.4% to 2.1%). Mortality due to Glasser’s disease remained low 1 year after introduction of the vaccine. We concluded that tbp gene analysis is a useful additional tool for identification of dominant and potentially protective strains of Haemophilus parasuis. | ResumenBuscamos vacunar de manera efectiva a las hembras de dos grandes sistemas de producción que presentaban enfermedad de Glasser muy severa en lechones destetados. El sistema A ó B surtían semanalmente 4000 ó 7000 lechones destetados a sus respectivos destetes individuales. Las lesiones sospechosas de las serosas se utilizaron para cultivar el Haemophilus parasuis y se hizo la serotipificación y genotipificación de los genes de unión a la transferrina tbpA y tbpB. La mayoría de los H parasuis cultivados pertenecían al serotipo 5, 12, ó no tipificables. Los productos específicos de PCR tbpA y tbpB se analizaron posteriormente mediante el polimorfismo de longitud de fragmento restringido. Éste indicó que las cepas dominantes pertenecían a los genotipos bb y hg (Granja A) y aa, bb, y kf (Granja B). Las cepas dominantes en los cultivos se expandieron y se inactivaron con formalina para utilizarlos como bacterinas autógenas en hembras, 5 y 2 semanas antes del parto. La mortalidad y la ganancia diaria de peso en los edificios donde residió la progenie de las hembras vacunadas (mortalidad de 0.5% a 0.9% para la población total) fueron menores que en los destetes que alojaron a los lechones de las hembras no vacunadas (1.4% a 2.1%). La mortalidad debida a enfermedad de Glasser se mantuvo baja 1 año después de la introducción de la vacuna. Concluimos que el análisis del gene tbp es una herramienta adicional útil para la identificación de las cepas dominantes y potencialmente protectoras de Haemophilus parasuis. | ResuméL’objectif de cet essai était de vacciner efficacement les truies dans deux organisations importantes aux prises avec la maladie de Glasser chez les porcs sevrés. Les organisations A ou B fournissaient hebdomadairement 4000 ou 7000 porcelets sevrés à leur pouponnière sur site unique respective. Les lésions suspectes sur les séreuses ont été cultivées pour Haemophilus parasuis, suivi du sérotypage et du génotypage des gènes tbpA et tbpB, gènes servant à lier la transferrine. La plupart des H parasuis cultivés appartenaient soit au sérotype 5 ou 12, ou étaient non-typables. Des analyses supplémentaires du polymorphisme des fragments de restriction ont été effectuées sur les produits de PCR spécifiques à tbpA et tbpB. Ces analyses indiquent que les souches dominantes appartiennent aux génotypes bb et hg (Ferme A), et aa, bb, et kf (Ferme B). Les souches dominantes sélectionnées ont été cultivées et tuées par traitement à la formaline en vue de leur utilisation comme bactérine autogène chez les truies 5 et 2 semaines avant la mise-bas. Les mortalités et le gain moyen quotidien dans les abris hébergeant la progéniture provenant des truies vaccinées (mortalité de 0.5% à 0.9% de la population totale) étaient inférieurs à ceux provenant de truies non-vaccinées (mortalité de 1.4% à 2.1%). Une année après l’introduction du vaccin, le taux de mortalité due à la maladie de Glasser est demeuré faible. En conclusion, l’analyse du gène tbp est un outil additionnel utile pour l’identification des souches dominantes et potentiellement protectrices d’Haemophilus parasuis. |
Keywords: swine, Haemophilus parasuis,
Glasser’s disease, vaccination, tbp genotype
Search the AASV web site
for pages with similar keywords.
Received: March 14, 2008
Accepted: July 25, 2008
Glasser’s disease is a common bacterial disease of pigs worldwide, caused by infection with Haemophilus parasuis. The various strains of H parasuis can be partly characterized via 15 heat-stable antigen serogroups; however, these have proved useful only for broad classifications of on-farm isolates, with many nontypable (NT) isolates also evident.1,2 It appears that different strains of H parasuis offer little cross-protection to pigs infected with other strains, and any protective pan-species antigen has yet to be identified. The development of genotyping schemes based on comprehensive polymerase chain reaction (PCR), such as repetitive intergenic analysis, or schemes based on particular genes, such as tbp or hsp60, has added to the useful tools for detailing the wide variety of on-farm H parasuis isolates.3-5 The advantage of schemes based on particular genes is that they may indicate genetic variation among potential virulence genes.4,5 Most farms have a stable flora of endemic H parasuis among their pigs, but this flora usually varies from farm to farm in its range of specific strains of H parasuis.1-3 Therefore, as swine farm systems enlarge and become more complex, the possibility of a single-strain commercial or autogenous vaccine covering all strains on such a farm becomes more difficult.6,7 Vaccine success may therefore rely on identifying the dominant strains in a farm prior to formulation of the vaccine mixture for that farm.
Haemophilus parasuis is carried in the tonsils and nasopharynx of most healthy adult pigs, and piglets are usually infected from their mother within a few days of birth.7,8 If these piglets also consume protective colostral antibodies, then many piglets may develop subclinical infections and acquire protective immunity.9 However, in situations where piglets consume insufficient colostral antibody, or where piglets are mixed post weaning with piglets derived from other farm sources that have a different flora of H parasuis, then those piglets will lack protective maternal antibody and may develop clinical signs of Glasser’s disease.8,9 In endemic herds, clinical signs will therefore usually include septicemia and respiratory signs approximately 2 weeks after weaning. Initial coughing and dyspnea due to bronchopneumonia is often followed by poor growth due to the distinctive fibrinous pleuritis and pericarditis associated with Glasser’s disease.7 These lesions and clinical signs usually respond poorly to antibiotic therapy, and many chronically affected piglets subsequently die or are euthanized in the late nursery period. In some situations, affected pigs may struggle on to the grower and finisher stages, with Glasser’s-associated pericarditis being recognized among slaughter-age lesions. On many farms, antibiotics are administered to recently weaned pigs to control septicemic spread, within each pig, of Glasser’s disease. This form of control has the advantages of not requiring detailed knowledge of maternal antibody status, nor of the dominant strains of H parasuis present on the affected farm. While specific farm sensitivity profiles are preferred, these are not always feasible.
In this study, we aimed to use the H parasuis tbp iron-binding virulence genotyping scheme in association with serotyping to select and develop autogenous vaccines containing the dominant farm strains of H parasuis to vaccinate the breeding herds of two large complex farm systems.
Case farm systems
Two farm systems in Australia were investigated. All procedures and decisions for animal care and euthanasia followed the current State Government Animal Welfare Advisory Committee guidelines (States of Victoria and New South Wales).
Farm A consisted of two adjacent-site breeder herds, A1 and A2, each of 4700 breeding females, supplying approximately 4000 three-week-old weaner pigs per week to a single adjacent on-site nursery facility. In this facility, pigs were mixed in curtain-sided sheds, each containing 10 to 20 pens. Pigs were removed from this nursery facility to grower-finisher sheds at 10 weeks old.
Farm B consisted of seven separate breeder herds, each of 500 to 2500 breeding females. The Glasser’s disease cases in progeny pigs apparently increased after an eighth 1200-gilt “start-up” breeding herd was added to the Farm B system. These eight breeder farms supplied approximately 7000 three-week-old weaner pigs per week to a 100-km distant, dedicated, single-site nursery facility that had numerous distinct sheds, not divided into pens, each filled with 400 pigs. In many instances, weaner pigs from different breeder-farm herds were mixed in a shed. Pigs were removed from this nursery facility to a separate facility of grower-finisher sheds at 10 weeks old.
The grower-finisher sheds for Farms A and B were located at separate sites > 20 km from other farm sites. Pigs were generally marketed at 100 kg live weight. All pigs were supplied with formulated diets derived from a single feed mill.
The breeding-herd origins of both farm systems consisted of a single source of synthetic Large White × Landrace crossbred pigs, with semen for artificial insemination supplied from a single boar-stud line. During this study, new genotypes were not introduced to the farms. Diets consisted of wheat-barley cereals with canola and other additives. In-feed antibiotic medications were supplied by veterinary prescription to diets destined solely for the target group of pigs. Both farm systems and these breeding herds had been free of clinical signs or other evidence associated with classical swine fever virus, porcine reproductive and respiratory syndrome virus, and pathogenic porcine circovirus type 2b virus for 10 years preceding and throughout this study, as monitored by ongoing autopsy, specific serological testing, and immunohistochemistry studies.
Laboratory analyses
Necropsies were performed on freshly dead or euthanized pigs to assess causes of death. In selected cases where fresh necropsy lesion material was available and the piglet had not been treated with antibiotics, attempts were made to culture serosal lesions typical of Glasser’s disease for H parasuis. The initial cultures were collected at a more intensive 4-week necropsy period (Period One; Figure 1) during the apparent disease peak, when a survey of all deaths (total mortality) was attempted. Swabs were collected from lesions of suspect Glasser’s disease serositis and occasionally bronchopneumonia, transported to the authors’ microbiology laboratory via Amies clear transport medium where necessary, and streaked within 60 minutes of collection onto a specific selective and enrichment medium for H parasuis culture (Haemophilus Isolation Agar with Bacitracin; BD, Franklin Lakes, New Jersey). A two-plate streak dilution of each sample was used to minimize overgrowth by contaminants, and separate early “nurse” cultures were used to assess V-factor requirements. Organisms were presumptively identified as H parasuis if they were V-factor dependent, positive for catalase, mannose, and saccharose, and negative for indole, urease, lactose, mannitol, raffinose, sorbitol, and CAMP.1,2 Antigenic serotyping of suspect isolates for heat-stable serotypes was performed by previously established methods.1,2 The antibiotic sensitivity of 18 selected isolates (Table 1) was estimated by the Kirby-Bauer disc diffusion technique conducted according to standard National Committee on Clinical Laboratory Standards guidelines.
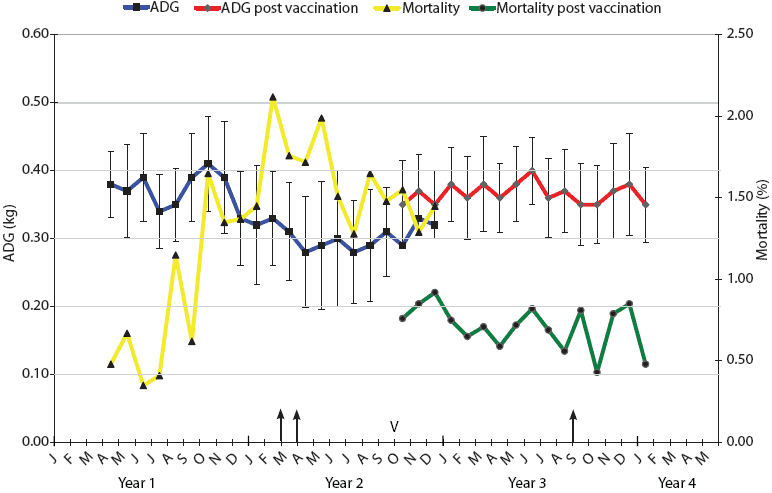
| Figure 1: Mortality and average daily gain (ADG) results
for weaner pigs (3 to 10 weeks old) in Farm A during the 3-year
span of this study. Intensive necropsy Periods One and Two (arrows)
were conducted in March-April of Year 2 and September of Year 3,
respectively. Vaccination of sows with autogenous Haemophilus
parasuis bacterin (V) began in October of Year 2. For a 3-month
period (October to December), progeny from both vaccinated and
nonvaccinated sows entered the weaner facility on an all-in,
all-out basis by shed. Mortality was calculated as the number of
deaths of weaner pigs expressed as a percentage of the total
population at the start of each month. ADG is expressed as kg/day ±
standard error, with measurements conducted on each incoming and
outgoing batch of pigs at the facility.
|
Table 1: Initial characteristics of Haemophilus parasuis isolates recovered from viscera of weaner pigs showing signs of Glasser’s disease in multi-site farm systems A and B*
* Necropsies were conducted during intensive mortality survey periods within clinical outbreaks, as described in Figures 1 and 2. Each isolate represents a single pig necropsy. † Individual isolate or group of isolates wholly or partly used for minimal inhibitory concentration determinations for a total of 18 tested isolates. NT = nontypable by antisera used. |
Genotyping of H parasuis
The genotype scheme used for this study is based on the tbpA and tbpB genes that encode for outer-membrane transferrin-binding proteins involved in iron uptake and survival by members of the Pasteurellaceae family of bacteria.6,10 These surface proteins are considered to have a significant impact on immunogenicity of Haemophilus and Actinobacillus members of this family.6,10,11 The tbpA and tbpB genotyping was performed according to previously established methods.10 Briefly, DNA was extracted from log-phase bacterial cultures by routine phenol-chloroform-isoamyl-alcohol methods. Polymerase chain reaction (amplification of tbp-specific sequences) was performed with the four PCR primers originally selected from published nucleotide sequences of tbpA and tbpB genes of Actinobacillus pleuropneumoniae (Genbank U16017) as described elsewhere.10 These primers were further verified for specificity against published nucleotide sequences for H parasuis tbpA and tbpB (Genbank AF336803 and AF378124, respectively).6
Restriction fragment length polymorphism (RFLP) analysis was performed on the H parasuis PCR-amplified products as described elsewhere.6,10 Briefly, tbpA PCR-amplified products were digested with DraI, NlaIV, AvaI, AsnI, TaqI, HinpI, BstEII, and NsiI, and tbpB PCR-amplified products were digested with RsaI, TaqI, AvaII, XmnI, BglII, and XbaI restriction endonucleases (New England Biolabs, Beverly, Massachusetts). The individual digested products were analyzed by agarose gel electrophoresis and for each enzymatic reaction, letters (eg, a,b,c) were assigned to each different restriction pattern observed for each gene, giving a final two-letter code for each isolate.
Selection and use of autogenous bacterins
The full list of serotypes and genotypes was developed for all the available isolates from each farm system, and major strains were identified. Selected strain cultures were expanded and finally grown separately for 6 hours in 2-L culture vessels containing tryptone yeast extract broth with added V-factor. Each batch was then tested for potency (colony forming units [cfu] per mL) and purity by titrating cultures onto the Haemophilus agar and routine aerobic and anaerobic plates. Each batch of pure culture was then inactivated with formalin blended into the final vaccine strain mixture (final concentration 0.2% vol/vol), and a commercial aluminum hydroxide gel adjuvant was added at 500 μg per mL. For each farm system, up to three strains were selected for incorporation into the final vaccine supply. Aliquots from each selected final batch mixture were decanted and pre-mixed into 100-mL bottles for on-farm use as specific autogenous bacterins in sows. Pregnant sows were dosed intramuscularly with 1 mL of vaccine per included strain (approximately 109 H parasuis per mL) at approximately 5 and 2 weeks prior to the predicted farrowing dates, aiming to enhance targeted H parasuis maternal antibodies available to their progeny at the time of weaning.
The performance of each new shed of sow-vaccinated progeny was compared with that of side-by-side sheds of nonvaccinated progeny over a 3-month vaccine-introduction period. A total of 45 and 150 sheds filled with weaner pigs derived from vaccinated sources were immediately adjacent to 60 and 148 sheds filled with pigs from nonvaccinated sources over this period for Farms A and B, respectively. Sheds were selected by convenience for assignment to each group on a whole-shed basis, ie, when sufficient pigs were available from a source to fill a whole shed. On-farm workers were blinded to shed assignments.
Monitoring of pig performance
The average daily gain (ADG) and mortality of each weekly batch of 4000 or 7000 weaner pigs (Farms A and B, respectively) were calculated by collation of the numbers of pigs per weaner shed in each batch on arrival and on departure, the number of days they were held in each shed, and the weights of each group of pigs at entry to and exit from the facility. Pigs were weighed in an electronic scale (accurate to 0.1 kg) holding 180 to 200 pigs, with adjustments made for daily mortality checks.
Pig performance continued to be closely monitored in vaccine-derived progeny for up to 1 year following introduction of the vaccines. Follow-up necropsy, cultures, and genotyping of remaining Glasser’s disease cases were collected after 1 year to establish whether new or minor strains were emerging into dominant status. Most cultures were collected at a second intensive necropsy period during apparent disease recovery (Period Two; Figures 1 and 2).
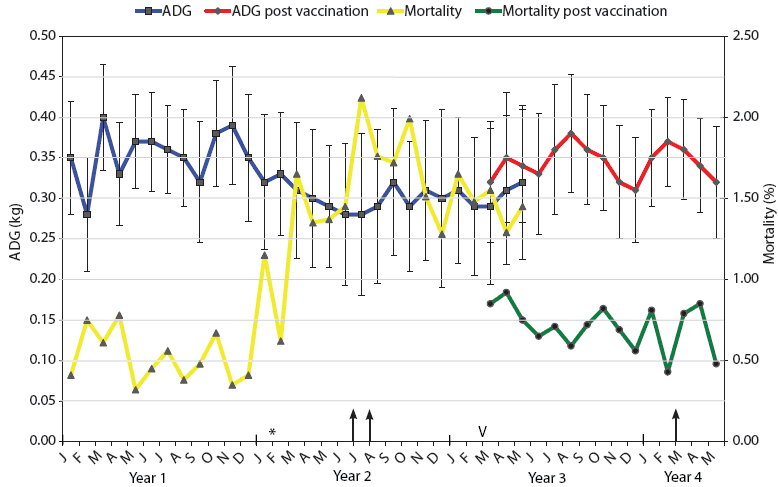
| Figure 2: Mortality and average daily weight gain results
for weaner pigs (3 to 10 weeks old) in Farm B during the 4-year
span of this study. The intensive autopsy Periods One and Two
(arrows) were conducted in July-August of Year 2 and March of Year
4, respectively. The time of addition of the new gilt-only source
of progeny for Farm B (February in Year 2) is indicated (*).
Vaccination of pregnant sows with autogenous Haemophilus
parasuis bacterin (V) began in March (M) of Year 3. For a
3-month period (March to May), progeny from both vaccinated and
nonvaccinated sows entered the weaner facility on an all-in,
all-out basis by shed. Mortality was calculated as the number of
deaths of weaner pigs expressed as a percentage of the total
population at the start of each month. ADG is expressed as kg/day ±
standard error, with measurements conducted on each incoming and
outgoing batch of pigs at the facility.
|
Disease occurrence post vaccination
The monthly mortality levels and ADG for the weaners (3 to 10 weeks old) over the years of this investigation for Farms A and B are indicated in Figures 1 and 2, respectively. The reasons for euthanasia and causes of death identified during necropsy Period One were 65% and 63% Glasser’s disease and 35% and 37% other causes for Farms A and B, respectively. The other major causes of death identified at these autopsies were mulberry heart disease, colibacillosis, and weaner starvation for both farm systems. During the rising mortality period, actions taken by farm managers, such as raising shed temperatures, increasing usage of amoxicillin in the feed and water supplies, and increased labor for appropriate attention to weaner care, had little noticeable effect, appearing to stabilize but not improve weaner losses.
Haemophilus parasuis characterization
During the initial case investigation, a total of 12 and 35 H parasuis isolates were cultured from serosal autopsy lesions from weaner pigs in farm systems A and B, respectively (Table 1).
Among the 18 selected isolates tested for antibiotic sensitivity, there was full sensitivity to tiamulin, florfenicol, amoxicillin, and penicillin. One strain from Farm A was resistant to tylosin and tilmicosin. Five strains were resistant to tetracycline, and 11 strains were resistant to lincomycin. As indicated in Table 1, the isolates chosen for this testing were selected from both farms and from a range of serotypes and genotypes.
Serotype results for the isolates are indicated in Table 1. Most were either serotype 5, 12, or nontypeable (NT) with the available rabbit antiserum against H parasuis heat-stable antigens. Specific PCR of the isolates produced 1.7-kb to 1.8-kb and 1.8-kb to 1.9-kb amplified fragments for tbpA and tbpB genes, respectively.
Restriction fragment length polymorphism characterization of these PCR fragments indicated that the most common tbp motifs for strains within farm system A were genotypes bb and hg (Table 1). Strains of these two genotypes were composed of H parasuis serotypes 5, 12, or NT (Table 1). Consideration of this serotype and genotype mix therefore suggested that strains of the bb and hg genotype were the dominant ones in this farm system. Isolates representing these two strains were therefore selected (bb, NT; hg, 12) and expanded for the autogenous vaccine developed for this farm system. These strains had originally been isolated from serosal lesions.
Similarly, the dominant strains within farm system B were considered to be those with genotypes aa, bb, and kf (Table 1). In this system, the aa and bb isolates were mainly serotype 5, with various strains having other combinations of serotype and genotype pattern. Isolates representing these three genotypes were therefore those selected (aa, 5; bb, NT; kf, NT) and expanded for the autogenous vaccine developed for this farm system. These strains had originally been isolated from serosal lesions. An NT isolate for the bb genotype was selected, rather than a serotype 5, because of superior in vitro growth.
Vaccine response
Autogenous vaccines were developed as specific bacterins for sows to provide maternal antibodies to the two or three dominant strains on Farms A and B, respectively. Monthly mortality and ADG in sheds containing progeny derived from vaccinated sows are shown in Figures 1 and 2. In the 3-month crossover period, the progeny derived from vaccinated sows were located in sheds adjacent to those housing progeny derived from nonvaccinated sows. There was a numerical difference in the levels of mortality and ADG for both farms during this crossover period, with lower mortality and higher ADG evident in progeny derived from vaccinated sows.
Follow-up case investigations
The reason for euthanasia and causes of death identified during autopsy Period Two, 1 year after the introduction of vaccine, were 16% and 18% Glasser’s disease and 84% and 82% other causes in Farms A and B, respectively. The other major causes of death identified at these autopsies remained mulberry heart disease, colibacillosis, and weaner starvation for both farm systems. Haemophilus parasuis was cultured as described from lesions in selected remaining cases of Glasser’s disease from both farms, with samples collected during Period Two. Isolates tested from these follow-up cases on Farm A (n = 3) were either nonserotypable bb or hg strains. Isolates from these follow-up cases on Farm B (n = 7) were either nonserotypable bb or serotype 14 de or serotype 15 ae strains.
Discussion
Glasser’s disease remains an important cause of losses and illness among pigs in so-called high-health herds, including those free of major immunosuppressive viruses, as described in these farm systems. On these study farms, we found several other common causes of weaner mortality, but the predominant health issue throughout was Glasser’s disease. The major source of the noted rise in Glasser’s disease-related mortality was apparently the decision to mix weaner pigs from a range of different breeding herds in these farm systems, particularly the mixture of progeny from a “start-up” herd in farm system B. Decisions affecting placements of weaner pigs are made on a continual basis on many large farm systems, which have to reconcile required outputs of finisher pigs with shifting breeder-herd numbers and status. This potential rise in Glasser’s and other transmissible diseases due to mixing of young weaner pigs may be an additional factor in the suggested success of three-site operations with strict management of flow of pigs throughout single-source sheds and sites.12 Where two- or three-site systems mix sources of progeny pigs into any weaner nursery site, then outbreaks such as described here may be confidently expected, due to the ubiquitous nature of multi-strain H parasuis.
Besides the long-term control option of strict pig flow controlled by three-site management, other useful control options for Glasser’s disease include the usage of antibiotics in weaners. Most isolates in this study showed good sensitivity to commonly used swine antibiotics such as tiamulin; these results are similar to those described elsewhere.13 Antibiotic usage for the control of Glasser’s disease and other common weaner-age infections, such as colibacillosis, has the advantages of being immediately applicable and of not requiring detailed knowledge of maternal antibody status nor of the dominant strains of H parasuis present on the affected farms. Antibiotic usage therefore remains an important tool for the welfare of weaner pigs, despite some pressures for its reduction.
A variety of vaccine approaches have been suggested for Glasser’s disease control. Unfortunately, it appears that individual strains of H parasuis offer little cross-protection to pigs infected with other strains, and any protective pan-species antigen has yet to be identified. Therefore, as farm systems consolidate and enlarge around the world, it is likely that Glasser’s disease will increase in incidence as weaner pigs with different strains of H parasuis are mixed. Because H parasuis infects piglets at a very young age, vaccine strategies have settled on either a live attenuated isolate aimed at early naso-pharyngeal colonization to then resist later colonization by more virulent H parasuis strains,14,15 or use of breeder female vaccination to supply targeted maternal antibody protection,8,16,17 as also suggested here. It is less likely that treating weaner or neonatal pigs with killed vaccines will provide adequate time for protection to develop before mixing of pigs at weaning. In addition, maternal antibody may interfere with the response to killed vaccines. 9
A range of commercial Glasser’s disease vaccines are available worldwide, each containing one or more strains of H parasuis. The serotype and genotype of the strain(s) in these products are often not widely available and may not reflect the full range or even part of the range of the dominant strains in farms wishing to apply a breeder vaccine strategy. Therefore, autogenous vaccines are a widely used strategy for Glasser’s disease, as these would aim to provide farm-specific strain protection. For this strategy to work properly, the range of strains evident on a farm should be adequately characterized and dominant strains selected prior to production of the bacterin strains to be then applied to the breeder farm.16 The aim of our study was to further clarify that this approach was valid, and we assess that it is indeed an approach that provided clear benefits for reduction of mortality and improved growth rates in the study farms. This was despite these farms having a complex and large-scale nature, with separate sites and breeder-farm complexes.
We chose the tbp genotyping scheme because the tbp iron-binding gene is considered an important virulence factor with a role in protective immunity development for the Pasteurellaceae.11 This approach therefore differs from some PCR-based methods that either characterize DNA segments located between repeated intergenic sequences (rep-PCR) or genes of unknown antigenic significance, such as hsp60 and 16S RNA.3,4 This is the first published study to then apply the tbp typing scheme to assist vaccine selection across large farm systems, and we found a range of genotypes among strains on each farm system, but with some dominant ones evident. Our results suggest that the tbp genotyping scheme combined with serotyping provides a very useful indicator of the dominant strains evident in large farms and therefore an indication of likely vaccine efficacy. Previous commercial vaccines and autogenous single-strain vaccine studies based on serotype-dominance selection have apparently worked well in simple farm systems.16,17 However, these vaccines would not be expected to provide comprehensive protection for larger farm systems with many strains present. Our study confirms the complexity of H parasuis and its vaccinal control, with each large farm system having a wide variety of strains identified by the mixture of sero- and genotyping methods. We chose to use a combination of these two methods to evaluate the dominant strains for selection of bacterin candidates. This level of strain characterization may be at the limit of appropriate complexity for autogenous vaccines. The apparent success of the vaccine contents, including the adjuvant involved, and the timing of vaccination, suggested that this vaccine approach was appropriate. It is expected that other farms in other locations would have different endemic strains of H parasuis, and other autogenous vaccine candidates would be required. It is also possible that use of more than three H parasuis strains would entail some difficulties in coordination of the expansion of individual strain cultures and resultant vaccine manufacture – autogenous vaccine batches are necessarily of a smaller and more temporary scale. It would be of great interest to compare the use of tbp typing with other previous typing systems, but this was outside the scope of this vaccine case study. This future work could also include comparison of tbp dominance in iron-restriction or sufficiency growth conditions.
While a considerable effort was made in selection, it is possible that selection and usage of more than two or three strains would have improved control of Glasser’s disease on these farms. Despite the vaccine program, autopsy of weaner mortality on Farm B 1 year later still found 18% of deaths were due to Glasser’s disease and that yet more strains were involved. A study of the dynamics of H parasuis strains in the face of antibiotic medications similarly found that the strains isolated from diseased pigs had apparently altered 1 year after commencement of therapy.18 In contrast, the remnant cases of Glasser’s disease on Farm A appeared to be due to strains similar to those present in the vaccine and may indicate that this level of control was at the limits of this vaccine approach.
Implications
- Glasser’s disease can form major outbreaks in recently weaned pigs mixed from different breeding-farm sources.
- Genotyping by tbp analysis is a useful tool for identification of dominant strains of H parasuis within a farm system.
- Vaccination of sows with the dominant farm strains of H parasuis is useful for control of subsequent Glasser’s disease incidence in weaner pigs.
Acknowledgements
We thank Connie Turni for laboratory assistance. We thank Neil Sammons of Intervet, who supervised the preparation of the vaccines. We also thank Rob Rosaia for organizing the original pig performance data collections.
References
1. Kielstein P, Rapp-Gabrielson VJ. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol. 1992;30:862–865.
2.Blackall PJ, Rapp-Gabrielson VJ, Hampson DJ. Serological characterisation of Haemophilus parasuis isolates from Australian pigs. Aust Vet J. 1996;73:93–95.
3. Oliveira S, Blackall PJ, Pijoan C. Characterization of the diversity of Haemophilus parasuis field isolates by serotyping and genotyping. Am J Vet Res. 2003;64:435–442.
4. Olvera A, Calsamiglia M, Aragon V. Genotypic diversity of Haemophilus parasuis field strains. Appl Environ Microbiol. 2006;72:3984–3992.
5. de la Puente-Redondo VA, Mendez JN, Blanco NG, Boronat NL, Gutierrez-Martin CB, Ferri EFR. Typing of Haemophilus parasuis strains by PCR-RFLP analysis of the tbpA gene. Vet Microbiol. 2003;92:253–262.
6. Nedbalcova K, Satran P, Jaglic Z, Ondriasova R, Kucherova Z. Haemophilus parasuis and Glasser’s disease in pigs: a review. Veterinarni Medicina. 2006;51:168–179.
7. Oliveira S, Pijoan C. Haemophilus parasuis: new trends in diagnosis, epidemiology and control. Vet Microbiol. 2004;99:1–12.
8. Kirkwood RN, Rawluk SA, Cegielsji AC, Otto AJ. Effect of pig age and autogenous sow vaccination of nasal mucosal colonization of pigs by Haemophilus parasuis. J Swine Health Prod. 2001;9:77–79.
9. Solano-Aguilar GI, Pijoan C, Rapp-Gabrielson VJ, Collins J, Carvalho LF, Winkelman N. Protective role of maternal antibodies against Haemophilus parasuis infection. Am J Vet Res. 1999;60:81–87.
10. de la Puente-Redondo VA, Blanco NG, Gutierrez-Martin CB, Mendez JN, Ferri EFR. Detection and subtyping of Actinobacillus pleuropneumoniae strains by PCR-RFLP analysis of the tbpA and tbpB genes. Res Microbiol. 2000;151:669–681.
11. Gonzalez GC, Yu RH, Rosteck PR, Schryvers AB. Sequence, genetic analysis and expression of Actinobacillus pleuropneumoniae transferrin receptor genes. Microbiology. 1995;141:2405–2416.
12. Fangman TJ, Tubbs RC. Segregated early weaning. Swine Health Prod. 1997;5:195–198.
13. Martin de la Fuente AJ, Tucker AW, Navas J, Blanco M, Morris SJ, Gutierrez-Martin CB. Antimicrobial susceptibility patterns of Haemophilus parasuis from pigs in the United Kingdom and Spain. Vet Microbiol. 2007;120:184–191.
14. Oliveira S, Batista L, Torremorell M, Pijoan C. Experimental colonization of piglets and gilts with systemic strains of Haemophilus parasuis and Streptococcus suis to prevent disease. Can J Vet Res. 2001;65:161–167.
15. Oliveira S, Pijoan C, Morrison R. Evaluation of Haemophilus parasuis control in the nursery using vaccination and controlled exposure. J Swine Health Prod. 2004;12:123–128.
16. Neilson R, Danielsen V. An outbreak of Glasser’s disease: studies on etiology, serology and the effect of vaccination. Nord Vet Med. 1975;27:20–25.
17. Smart NL, Miniats OP. Preliminary assessment of a Haemophilus parasuis bacterin for use in specific pathogen free swine. Can J Vet Res. 1989;53:390–393.
18. Olvera A, Cerda-Cuellar M, Nofrarias M, Revilla E, Segales J, Aragon V. Dynamics of Haemophilus parasuis genotypes in a farm recovered from an outbreak of Glasser’s disease. Vet Microbiol. 2007;123:230–237.