Payne BJ, Clark S, Maddox C, et al. Achromobacter xylosoxidans in extended semen causes reproductive failure in artificially inseminated sows and gilts. J Swine Health Prod. 2008;16(6):316–322
| Case report | Peer reviewed |
Cite as: Payne BJ, Clark S, Maddox C, et al. Achromobacter xylosoxidans in extended semen causes reproductive failure in artificially inseminated sows and gilts. J Swine Health Prod. 2008;16(6):316–322.
Also available as a PDF.
SummaryAchromobacter xylosoxidans, a Pseudomonas-like bacterium, contaminated a boar stud laboratory’s water distillation and delivery system that was used for extension of raw semen for artificial insemination. In sow herds that used the contaminated semen, conception rates decreased and vulvar discharges, culled sows, and nonproductive sow days increased. Thorough diagnostic testing revealed that A xylosoxidans was the etiological agent of endometritis and subsequent reproductive failure in these sows and gilts. This is the first known report of female reproductive failure attributed to A xylosoxidans. Routine cleaning and control measures to reduce bacterial growth in the extender ultimately failed to eliminate A xylosoxidans. Following short-term elimination of A xylosoxidans from the extended semen, reproductive parameters in the sow herds returned to their previous levels. | ResumenEl Achromobacter xylosoxidans, una bacteria similar a la Pseudomonas, contaminó el sistema de suministro y destilación de agua que se utilizaba para la dilución de semen en un laboratorio de un centro de inseminación artificial. En las piaras que utilizaron el semen contaminado, el índice de concepción disminuyó y las descargas vulvares, las hembras desechadas, y los días no productivos aumentaron. El diagnóstico reveló que el A xylosoxidans era el agente etiológico de la endometritis y las subsecuente falla reproductiva en estas hembras y primerizas. Este es el primer reporte conocido de falla reproductiva en hembras atribuido al A xylosoxidans. Las medidas de control y la limpieza rutinarias para reducir el crecimiento bacteriano en el diluyente falló en eliminar el A xylosoxidans. Después de la pronta eliminación del A xylosoxidans del semen diluido, los parámetros reproductivos regresaron a sus niveles previos. | ResuméAchromobacter xylosoxidans, une bactérie apparentée à Pseudomonas, a contaminé le système de distillation et de distribution d’eau du laboratoire d’une verraterie. Cette eau servait à diluer la semence brute utilisée pour insémination artificielle. Dans les troupeaux de truies où la semence contaminée a été utilisée, on observa une diminution des taux de conception, et une augmentation du nombre de truies présentant un écoulement vulvaire, du nombre de truies réformées, et du nombre de jours-truies non productives. Une analyse minutieuse a démontré qu’A xylosoxidans était l’agent étiologique d’endométrite et des problèmes de reproduction subséquents chez les truies et cochettes. Il s’agit du premier rapport faisant état de problèmes reproducteurs chez des femelles associés à A xylosoxidans. Un nettoyage de routine et des mesures de contrôle pour réduire la croissance bactérienne dans le dilueur n’ont pas réussi à éliminer A xylosoxidans. Suite à l’élimination à court terme d’A xylosoxidans de la semence diluée, les paramètres de reproduction des troupeaux affectés sont revenus à leurs niveaux antérieurs. |
Keywords: swine, semen, vulvar discharge, endometritis, Achromobacter xylosoxidans
Search the AASV web site
for pages with similar keywords.
Received: December 10, 2007
Accepted: January 23, 2008
Poor semen quality is one of the primary causes of female reproductive failure in animals, including swine. Sperm motility, sperm morphology, and concentration of the semen dose produced are the parameters commonly used to assess semen quality. These three parameters are easily and routinely evaluated at commercial boar studs throughout the swine industry. However, other potential indicators of poor semen quality may not be evaluated in every semen batch. Pathogenic and opportunistic bacteria are threats to semen quality that may cause reproductive failure in sows.1 Some of these organisms may not be considered pathogens in the pig. Reicks2 showed that, among 15 bacterial species isolated from semen, the water system was the source of the three most common genera, Alcaligenes, Pseudomonas, and Burkholderia. Other potential sources include the ejaculate itself, environmental contamination, and tubing or equipment used in the extension process. In 23 field investigations, Althouse et al1 found that Alcaligenes xylosoxydans was the bacteria most frequently isolated, followed by Burkholderia cepacia, Enterobacter cloacae, Escherichia coli, Serratia marcescens, and Stenotrophomonas maltophilia. In a later retrospective study of 250 samples, Althouse and Lu3 found that A xylosoxidans was present in 10.3% of cases, third behind Enterococcus species (20.5%) and S maltophilia (15.4%).
Achromobacter xylosoxidans is a Pseudomonas-like bacterium that has been identified as a fairly common semen contaminate.1,2 This organism, formerly classified as Alcaligenes xylosoxidans subspecies xylosoxidans, is an aerobic, gram-negative, nonfermentative, flagellated, rod-shaped bacterium.4 It is rarely isolated as the primary pathogen in clinical cases, but causes human nosocomial infections in a dose-dependent manner.4 Members of the genus Pseudomonas produce biofilms, making them resistant to common disinfection methods and difficult to eliminate from a water system.
System description
Throughout the period of this case study, a 180-head boar stud located in the Midwest United States served a 21,000-sow system and several other smaller sow herds in northern Illinois. The sow system consisted of two genetic nucleus farms, two gilt multipliers, five commercial sow centers, two gilt breeding-gestation sites, and one commercial farrow-to-finish sow farm. The breeding herd was housed in industry-acceptable gestation stalls during breeding and gestation and was cared for humanely under the guidance of a veterinarian and in accordance with the guidelines of the National Pork Board.5
Boar stud case description
Discovery of contamination
In the fall of 2005, bacterial contamination was discovered in extended semen doses by the herd veterinarian during routine culturing of samples submitted for third-party analysis at the Porcine Andrology Laboratory at the University of Illinois College of Veterinary Medicine. The primary contaminant, A xylosoxidans, was consistently recovered. Ralstonia picketti, a related organism, was also recovered sporadically. During this same time period, third-party analysis showed no negative effects on semen characteristics such as motility and morphology.
Microbial susceptibility
Although there were no apparent ill-effects to the sperm or clinical female reproductive consequences early in the clinical investigation, the herd veterinarian considered bacterial contamination of the semen to be unacceptable.
Three accredited university laboratories were consulted. Laboratory A used a 32-well substrate system (ARES system; Trek Diagnostics, Cleveland, Ohio) and Laboratories B and C used 96-well substrate systems (Biolog MicroID; Biolog, Hayward, California, and API 20E, BioMerieux, Hazelwood, Missouri, respectively) to identify the genus and species of Pseudomonas-like organisms. Laboratory A identified A xylosoxidans less often than Laboratories B and C. In order for the herd veterinarian to be confident in a single laboratory for this specific problem, forward and reverse genetic sequencing of the 16s-23s rRNA genes were performed (Lloyd Carver Biotechnology Institute, University of Illinois, Urbana-Champaign, Illinois) on a typical isolate from Laboratory B. Percent genetic similarity in these genes, compared with sequences deposited in the National Center for Biotechnology Information database for A xylosoxidans, was 99% (forward) and 100% (reverse). As a result, Laboratory B, the University of Illinois College of Veterinary Medicine Diagnostic Laboratory, was utilized for culturing and susceptibility testing of semen for this boar stud. In susceptibility tests based on minimum inhibitory concentration (MIC) testing using Clinical and Laboratory Standards Institute M31-A2 Guidelines,6 A xylosoxidans was resistant to ceftiofur, clindamycin, erythromycin, florifenicol, gentamicin, neomycin, penicillin, spectinomycin, sulfachlorpyridazine, sulfadimethoxine, sulphathiazole, tiamulin, tilmicosin, and trimethoprim-sulfamethoxazole. It was susceptible to ampicillin, chlortetracycline, enrofloxacin, and oxytetracycline. The susceptibility pattern had no interpretative guidelines for tylosin and danofloxacin. Gentamicin is included in the Beltsville Thawing Solution extender (IMV USA, Maple Grove, Minnesota) used in the boar stud laboratory. Ceftiofur (Naxcel; Pfizer Animal Health, New York, New York) was added to the extender at 300 mg per 1000 mL. Both antibiotics are used to increase the shelf-life of collected semen by reducing the bacterial pathogen load. Tri-Cell extender (IMV USA), which contains amoxicillin, gentamicin, and tylosin, was also occasionally used in the laboratory. Susceptibility to amoxicillin was not tested.
Source of bacterial contamination
Identifying the source of A xylosoxidans was the first step in trying to eliminate the organism from the extended semen doses. Multiple cross-sectional samples of raw semen and their corresponding single-sire extended semen doses were cultured on Remel Columbia Blood Agar (Remel, Lenexa, Kansas). Lack of A xylosoxidans growth from raw semen cultures and repeatable growth from extended semen samples identified the extension process as the source of contamination.
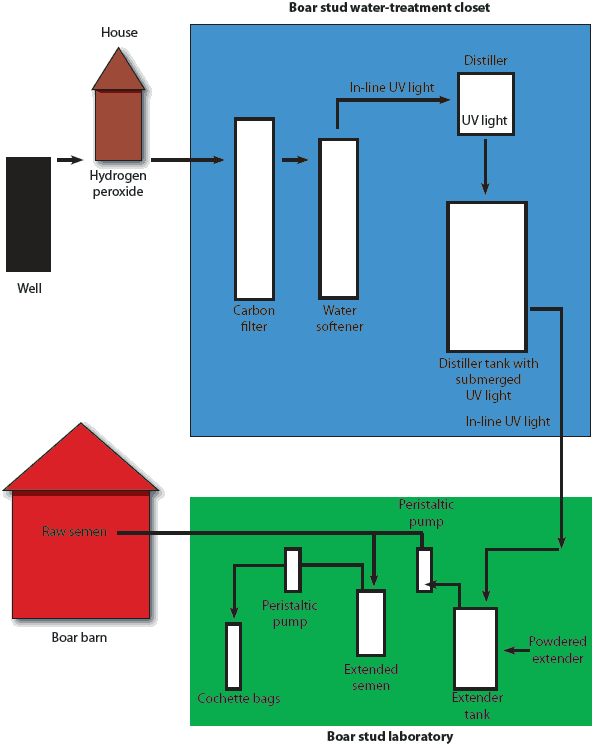
Subsequently, the well water used in semen extension was treated through a multi-step process (Figure 1). Prior to changes that were made because of contaminated semen, water had been pumped from a well and treated with hydrogen peroxide (1 mg per L of water from an 11% hydrogen peroxide stock solution), had flowed through a carbon filter, entered a water softener, and passed through an in-line ultraviolet light (Sterilight Silver, Model SQ-PA; R-Can Environmental Inc, Guelph, Ontario, Canada) into a single distiller at a rate of 5.6 L per minute. The distilled water dripped into a holding vat with a submersible ultraviolet light (253.7 nm, 30 mJ per cm2, 10W bulb). It was then pumped through a single flexible 0.64-cm opaque tube with a drop line entering an extension vat where powdered extender was added to the warm water. A stir rod aided in reconstituting and mixing the extender solution, which was added to raw semen via a peristaltic pump (Masterflex; Cole-Parmer Instrument Co, Vernon Hills, Illinois) and disposable 0.64-cm transparent tubing. A second peristaltic pump and disposable tubing pumped the extended semen into cochette bags.
| Figure 1: Schematic of laboratory closet and critical
control points to eliminate contamination of water used to make
semen extender for a 180-head boar stud.
|
Early in the investigation process, A xylosoxidans was cultured from 11 of 28 water and culturette swabs (39%) from the various sections of the water-treatment flow. The flexible tubing downstream from the water softener and all critical control points thereafter were culture-positive for A xylosoxidans during the investigation, but all critical control points upstream from the water softener were culture-negative.
Attempted removal of bacteria
Ceasing use of water from the boar stud for extension of semen was the first control measure in late 2005. Bottled distilled water from a commercial water supply company was used during downtime of the water distillation system. The distilled water was sporadically culture-positive for R picketti, and all samples tested (n = 5) were culture-negative for A xylosoxidans. The water system was emptied and a 50% bleach solution was added to the distiller tank and drained through the remaining lines and drops, remaining in the lines overnight. After several rinses with commercially purchased and culture-negative distilled water, the lines were drained and then were allowed to dry for 2 weeks. At the end of this time, the treatment system was returned to use and two water samples were collected weekly from the end of the treatment line. All samples were culture-negative for A xylosoxidans. For the following 3 weeks, water that was culture-negative for aerobic bacteria was used for extension of semen. During the third week, A xylosoxidans was again identified as a contaminant in water samples. The system was again shut down and the described cleaning process was repeated. In addition, internal and external surfaces, connectors, and valves were sprayed with 70% ethanol. Within 2 weeks, A xylosoxidans was again cultured from water samples.
A new distiller, distiller tank, second in-line UV light, and new disposable lines were purchased and installed during weeks seven and eight of 2006. On week nine, the new system’s water was immediately used for extension of semen. During weeks 10 and 11, water cultured negative, but on week 12, samples cultured positive for A xylosoxidans. Nevertheless, water from the distillation system continued to be used in semen extender, as no problems were reported from the sow system, and in-house and third-party analysis showed no degeneration of semen quality.
Sow herd case description
Reproductive failure in several sow herds
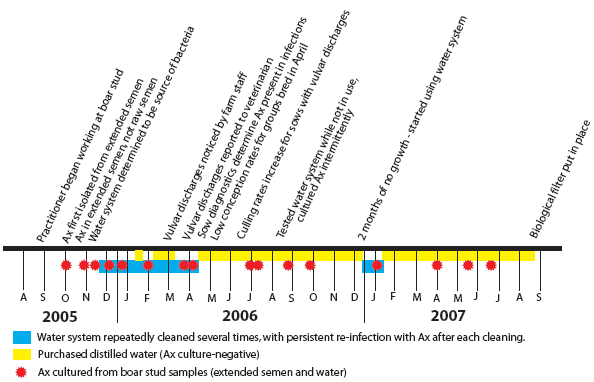
During the fall of 2005 and early 2006 (Figure 2), there were no indications of reproductive failure in the sow system. Conception rates, farrowing rates, pigs born alive, and numbers of mummies and stillborns remained stable. During week 14 of 2006, one sow farm reported white purulent vulvar discharges from sows and gilts within 3 to 19 days of breeding. Further investigation revealed that all sow farms in the system were affected. Discharges had been noticed by farm employees as early as week 10, but had not been reported to the herd veterinarian. The prevalence of vulvar discharges was 8% to 15% for breeding groups within 3 weeks post breeding. By week 15, a full investigation made it apparent that contaminated semen was the cause of discharges.
| Figure 2: Timeline of identification of Achromobacter
xylosoxidans (Ax) in the water used to make semen extender at a
180-head boar stud and clinical signs in the 21,000-sow system
supplied with semen from the stud.
|
Clinical signs
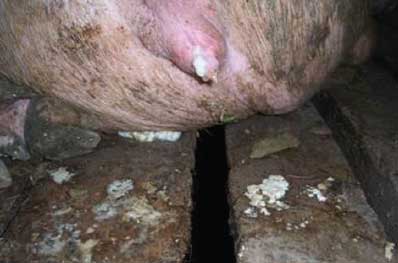
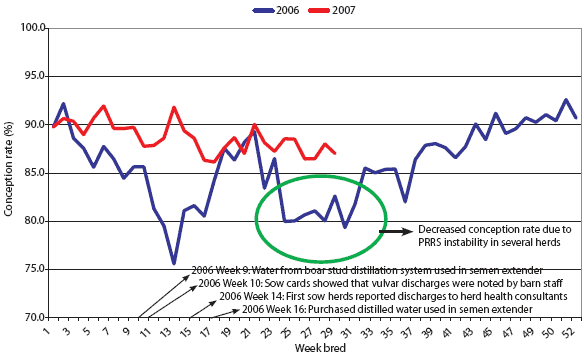
The first clinical sign in the sow herds, subjectively observed (not recorded), was decreased feed intake, followed by white purulent vulvar discharges (Figure 3) occurring 3 to 19 days after insemination. Over 50% of sows and gilts identified with vulvar discharges were negative at pregnancy check with real-time ultrasound 4 to 5 weeks post artificial insemination. From weeks 10 to 17 in 2006, there was a drop in average conception rate for the sow farms in the 21,000-head sow system (Figure 4). Conception rates returned to normal at week 18. Purchased distilled water was used in semen extender beginning in week 16, but some females that had discharges during weeks 10 to 12 were rebred during weeks 16 and 17, which may account for the lag time between use of uncontaminated extender and return to normal conception rate. A drop in conception rate during weeks 25 to 37 of 2006 was associated with an outbreak of PRRS that caused reproductive failure in several sow farms in the system, rather than with bacterial contamination of semen.
| Figure 3: Purulent vulvar discharge from sow artificially
inseminated with semen contaminated with Achromobacter
xylosoxidans.
|
| Figure 4: Average conception rates across a 21,000-sow
system in which semen doses used for artificial insemination were
contaminated with Achromobacter xylosoxidans. Semen extender
made using water from the distillation system in the boar-stud
laboratory (shown in Figure 1) was the source of contamination.
Samples from the distillation system became culture-negative for A xylosoxidans after extensive cleaning and disinfection of
the system. During treatment of the distillation system, purchased
distilled water that was culture-negative for A xylosoxidans was used in semen extender.
|
Necropsy of affected and unaffected sows
Seven sows from four different sow farms were submitted live to the University of Illinois College of Veterinary Medicine for necropsy and diagnostic testing 5 to 21 days post breeding. Five sows had vulvar discharges (Figure 3). The other two (controls) were normal-appearing sows with no evidence of vulvar discharge.
Endometritis was diagnosed in the five sows with vulvar discharges. In each case, the lumen of the uterus contained approximately 10 to 30 mL of white, caseous material and the endometrium appeared red, edematous, and thickened. The submucosa was congested and edematous. Neutrophils infiltrated the epithelium, submucosa, and endometrial glands and were found in the lumen of the uterus. Exocystosis of neutrophils was evident. The endometrial glands were often replaced by neutrophils. Inguinal lymph nodes were hyperplastic. Tonsil sections contained large numbers of neutrophils, cellular debris, and a few macrophages. Bacilli were present in the lumen and epithelium of the uterus. In four of the five sows, A xylosoxidans was isolated from the uterus. In one sow, A xylosoxidans was isolated from the kidney. No evidence of reproductive-system inflammation was observed in the two control sows, and A xylosoxidans was not isolated from the tissues. Among these seven sows, Escherichia coli was isolated from two sows with vulvar discharges and one that had no vulvar discharge; therefore, E coli was not considered to be the etiological agent of endometritis.
Intrauterine cultures
Intrauterine swabs (Double Guarded Culture Swabs; Jorgenson Laboratories, Inc, Loveland, Colorado) were used to monitor for bacterial growth in sows with vulvar discharges. Uterine swabs were collected during the summer of 2006 from nine sows with and four sows without vulvar discharges, and were submitted to the diagnostic laboratory of the University of Illinois for culture. Achromobacter xylosoxidans was not cultured from swabs collected from sows without vulvar discharges, but was cultured from swabs collected from six of nine sows with vulvar discharges.
Ruling out other possible causes of vulvar discharges
Many potential causes of endometritis were ruled out because of the widespread discharge and conception-rate problem in the system. The affected herds were located in three separate counties. Feed was provided from five separate feed mills with numerous independent feed-ingredient sources. The nearly simultaneous occurrence of clinical signs in the sow herds made it very unlikely that management or nutrition was the cause.
Breeding technique and semen handling were evaluated in several of the sow herds when clinical signs were first noticed. There was no indication of unhygienic techniques or improper timing of AI.
Predisposing factors causing immune-system dysfunction in the breeding herd were evaluated. Routine mycotoxin testing results from the feed mills were requested and mycotoxins were not detected. Feed samples submitted monthly from five of the sow herds showed no abnormalities in diet composition, and mycotoxins were not identified. All sow herds were PRRS-positive, including the genetic and multiplier herds. Arguably, as PRRS virus may cause immunosuppression,7 endemic PRRS might be a predisposing factor for bacterial endometritis.
Attempted control measures in sows
Original treatments were based on susceptibility profiles of both the semen and uterine isolates. The susceptibility patterns of the uterine isolates were identical to that of the isolate cultured from the extended semen. Parenteral (oxytetracycline), intrauterine (oxytetracycline), and oral (chlortetracycline) antibiotic protocols were used to treat infected sows, but were deemed ineffective. Therefore, parenteral ceftiofur, penicillin, and tylosin were also utilized, although the isolates were resistant to ceftiofur. There was no response to treatment. Cull rates increased because of sows and gilts with discharges that were found not pregnant and were entered into the data collection system as “uterine infection” culls by the managers at all nine sow farms. There were no reports of rejected carcasses or significant cut-out from the slaughter facilities.
Discussion
Although A xylosoxidans has not previously been reported to cause reproductive failure in sows, it appeared to be the cause of severe endometritis and subsequent reproductive failure in sows and gilts in this system. Endemic PRRS was most likely a predisposing factor for endometritis. It is possible that the clinical signs and negative effect on conception rate would not have been as severe in a PRRS-negative system.
The thorough diagnostic workup on females with and without discharges provided strong evidence that A xylosoxidans was the etiological agent of endometritis in this sow system. Further investigations using intrauterine inoculation in healthy gilts and subsequent isolation of A xylosoxidans from vulvar discharges needs to be accomplished to satisfy Koch’s postulates.
In this case, the source of A xylosoxidans was extended semen from the boar stud. This is one of the most common bacterial species isolated from extended semen. Although its isolation in this boar stud was not unique, the resilience of the bacteria in the water-treatment system was surprising. Thorough cleaning, disinfecting, and lengthy drying periods did not eliminate A xylosoxidans. A seeding source was suspected but was not discovered.
The boar-stud laboratory personnel are currently experimenting with biofilters placed on the ends of water treatment lines (Figure 1). The Porcine Andrology Laboratory at the University of Illinois College of Veterinary Medicine is continuing to investigate this case to better understand the growth dynamics of A xylosoxidans and other potential control measures in the water system, not only for this organism, but for other bacterial contaminants as well.
In this boar stud, semen was not routinely cultured before September 2005. It is suspected that bacteria, including A xylosoxidans, had contaminated the semen earlier and might have been the cause of reproductive failure in sows previously. Semen, extended semen, and laboratory equipment should be routinely cultured to quickly identify bacterial contamination. In this case, weekly culturing of raw ejaculates, extended semen, and water used for extension was implemented, as well as monthly culturing of the laboratory environment.
The intrauterine swabbing technique continues to be utilized to monitor uterine discharges. Further information is needed and is being collected on the potential negative effects of this technique on females that have discharges but are still pregnant. As of June 2008, there appeared to be no negative effects.
In order to avoid a delay in reporting sow herd issues such as vulvar discharges, weekly reporting of key issues has been implemented in this system, including conception rates, vulvar discharges, and abortions, which allows rapid collection of diagnostic samples to determine causes of reproductive failure.
Implications
- Achromobacter xylosoxidans in extended porcine semen can cause endometritis, vulvar discharge, and serious and economically devastating female reproductive failure.
- This case emphasizes the importance of water quality and preventing use of bacteria-contaminated water for semen extension.
- More work is needed to better understand the reproductive dynamics and control measures in a herd that uses extended semen contaminated with A xylosoxidans.
- Systematic and continuous communication among farm staff, management, and veterinary health services concerning reproductive health problems is essential to achieve rapid diagnoses and timely initiation of effective treatments and preventive measures.
References
1. Althouse GC, Kuster CE, Clark SG, Weisiger RM. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology, 2000;53:1167–1176.
*2. Reicks DL, Bacterial contamination and semen quality. Proc Leman Swine Conf. St Paul, Minnesota. 2003;169–170.
3. Althouse GC, Lu KG. Bacteriospermia in extended porcine semen. Theriogenology, 2005;63:573–584.
4. Tsay RW, Lin LC, Chiou CS, Liao JC, Chen CH, Liu CE, Young TG. Alcaligenes xylosoxidans bacteremia: clinical features and microbiological characteristics of isolates. J Microbiol Immunol Infect. 2005;38:194-199.
*5. National Pork Board. Animal Welfare. Publications and Programs. Available at: www.pork.org/PorkScience/. Accessed 5 July 2008.
6. Clinical and Laboratory Standards Institute (Formerly NCCLS). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standards. 2nd ed. Document M31-A-2;22(6). Wayne, Pennsylvania: National Committee for Clinical Laboratory Standards; 2002.
7. Benfield DA, Collins JE, Dee SA, Halbur PG, Joo HS, Lager KM, Mengeling WL, Murtaugh MP, Rossow KD, Stevenson GW, Zimmerman JJ. Porcine reproductive and respiratory syndrome. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:201–232.
*Non-refereed references.