Patterson AR, Karriker LA, Apley MD, et al. Plasma concentrations of sodium salicylate in nursery pigs treated orally. J Swine Health Prod. 2007;15(3):146–151
| Original research | Peer reviewed |
Cite as: Patterson AR, Karriker LA, Apley MD, et al. Plasma concentrations of sodium salicylate in nursery pigs treated orally. J Swine Health Prod. 2007;15(3):146–151.
Also available as a PDF.
SummaryObjectives: To determine stability of acetylsalicylic acid (ASA) and sodium salicylate (SS) stock solutions, and to determine plasma concentrations of SS in swine after administration in drinking water. Materials and methods: Stock solutions of liquid ASA and SS products were placed in a nursery environment for 24 hours (Trial One). Salicylate concentrations were measured at 0, 8, 16, and 24 hours using high pressure liquid chromatography (HPLC). In Trial Two, SS was metered into the drinking water of four groups of pigs in a commercial nursery, at stock solution concentrations of 2268 mg per L (T1), 4913 mg per L (T2), 9827 mg per L (T3), and 19,654 mg per L (T4). Controls received nonmedicated water. Plasma salicylate concentrations in 10 pigs per group were measured at 0, 24, 60, and 72 hours. Results: Salicylate concentration ranges of stock solutions in Trial One were 4.04 to 4.61 g per L (ASA) and 8.19 to 9.34 g per L (SS). In Trial Two, mean plasma salicylate concentration ranges for treated groups over the 72-hour study were 0.17 to 0.41 mg per L (T1), 0.03 to 1.28 mg per L (T2), 0.44 to 1.41 mg per L (T3), and 0.62 to 7.22 mg per L (T4). Mean concentrations increased at 24 hours post study initiation, then decreased for all treated groups at 60 hours. Implications: Solubilities of ASA and SS products differ. Sodium salicylate administered via a water-medication system reaches measurable plasma concentrations in nursery pigs. Consistency of dosing may be variable. | ResumenObjetivos: Determinar la estabilidad de soluciones madres de ácido acetilsalicílico (ASA por sus siglas en inglés) y salicilato de sodio (SS por sus siglas en ingles), y determinar las concentraciones en plasma de SS en cerdos después de la administración en el agua de bebida. Materiales y métodos: Las soluciones madres SS y ASA líquidos se colocaron en un medioambiente de destete por 24 horas (Prueba Uno). Las concentraciones de salicilato se midieron a las 0, 8, 16, y 24 horas utilizando cromatografía líquida de alta presión (HPLC por sus siglas en inglés). En la Prueba Dos, el SS se midió en el agua de bebida de cuatro grupos de cerdos de un destete comercial, en concentraciones de soluciones madre de 2268 mg por L (T1), 4913 mg por L (T2), 9827 mg por L (T3), y 19,654 mg por L (T4). Los controles recibieron agua no medicada. Se midieron las concentraciones en plasma de salicilato en 10 cerdos por grupo a las 0, 24, 60, y 72 horas. Resultados: Los rangos de concentración del salicilato de las soluciones madre en la Prueba Uno fueron de 4.04 a 4.61 g por L (ASA) y 8.19 a 9.34 g por L (SS). En la Prueba Dos, los rangos de concentración promedio del salicilato en plasma para los grupos tratados durante el estudio de 72 horas fueron de 0.17 a 0.41 mg por L (T1), 0.03 a 1.28 mg por L (T2), 0.44 a 1.41 mg por L (T3), y 0.62 a 7.22 mg por L (T4). Las concentraciones promedio incrementaron a las 24 horas después del inicio del estudio, y luego disminuyeron a las 60 horas en todos los grupos tratados. Implicaciones: La solubilidad de los productos de ASA y SS difiere. El salicilato de sodio administrado a través de un sistema de medicación por agua alcanza concentraciones medibles en plasma en cerdos de destete. La consistencia de la dosis puede ser variable. | ResuméObjectifs: Déterminer la stabilité de solutions mères d’acide acétylsalicylique (ASA) et de salicylate de sodium (SS), et mesurer les concentrations plasmatiques de SS chez des porcs suite à l’administration dans l’eau de boisson. Matériels et méthodes: Des solutions mères d’ASA et de SS ont été mises dans l’environnement d’une pouponnière pour une durée de 24 heures (Expérience 1). Les concentrations de salicylate ont été mesurées par chromatographie liquide à haute pression (HPLC) après 0, 8, 16, et 24 heures. Lors de l’Expérience 2, le SS a été administré dans l’eau de boisson à quatre groupes de porcs dans une pouponnière commerciale à des concentrations de 2268 mg par L (T1), 4913 mg par L (T2), 9827 mg par L (T3) et 19,654 mg par L (T4). Les animaux témoins ont reçu de l’eau non-médicamentée. Les concentrations de salicylate plasmatique chez 10 porcs par groupe ont été mesurées à 0, 24, 60, et 72 heures. Résultats: Les plages de concentration de salicylate des solutions stocks dans l’Expérience 1 étaient de 4.04 à 4.61 g par L (ASA) et de 8.19 à 9.34 par L (SS). Dans l’Expérience 2, les plages de la concentration plasmatique moyenne de salicylate pendant la période de 72 heures de l’étude étaient de 0.17 à 0.41 mg par L (T1), 0.03 à 1.28 mg par L (T2), 0.44 à 1.41 mg par L (T3), et 0.62 à 7.22 mg par L (T4). Les concentrations moyennes augmentaient 24 heures après le début de l’expérience, puis diminuaient à 60 heures pour tous les groupes traités. Implications: La solubilité de l’ASA et du SS diffère. Le salicylate de sodium administré à l’aide d’un système permettant la médication par l’eau atteint des concentrations plasmatiques mesurables chez des porcelets en pouponnière. L’uniformité du dosage peut être variable. |
Keywords: swine, pharmacology,
anti-inflammatory drugs, aspirin
Search the AASV web site
for pages with similar keywords.
Received: September
2, 2005
Accepted: September
14, 2006
Acetylsalicylic acid (aspirin; ASA) is commonly used as an analgesic, antipyretic, and anti-inflammatory drug1 in animals, although the Food and Drug Administration Center for Veterinary Medicine has never approved ASA for these purposes.2 Aspirin and sodium salicylate (SS) appear to be widely used in food-animal production due to the inexpensive cost, over-the-counter availability,3 and the lack of anti-viral drugs approved for use in food animals (Dr Mike Apley, personal communication, 2004). Additionally, clinical outbreaks of viral diseases, such as porcine reproductive and respiratory syndrome and swine influenza, have not been adequately controlled with current vaccination protocols (ie, outbreaks are still common). Consequently, producers commonly use ASA or SS products to offset the performance losses associated with pyrexia and anorexia. The reported effectiveness of using aspirin in this manner is varied and based on anecdotal information.
The major mechanism by which aspirin exerts both its beneficial and negative effects is inhibition of prostaglandin synthesis.4-6 Aspirin irreversibly inhibits both cyclooxygenase-1(COX-1) and COX-2 by acetylating a serine at the active site. Although inhibition of COX by ASA has been well elucidated, salicylate (the metabolic derivative of ASA) shows no practical anti-COX activity, although it still has anti-inflammatory actions.4 While the exact mechanism of action for SS’s anti-inflammatory effects has not been determined, multiple theories have emerged. Amman and Peskar (2002) 7 have reviewed possible mechanisms.
Very little reported information exists on the dosage or plasma concentrations achieved in swine following administration of ASA or SS via a water medication system. For pain in swine, the USP Veterinary Pharmaceutical Information Monographs2 recommends an oral dose of 10 mg per kg of ASA per 6 to 8 hours (30 to 40 mg per kg per day). It is suggested that this dose be increased to 2 mg per kg per hour (48 mg per kg per day) if a “water supply” is used for administration. The corresponding water dose for SS would be 1.8 mg per kg per hour or 43 mg per kg per day. However, the type of water medication system the ASA dosage applies to is not specified, nor are references cited concerning the assumptions made in calculation of this dosage (eg, water consumption per hour, equal opportunity to drink). Other references list similar recommendations.1,5,8 No recommendations of dosages for antipyretic or anti-inflammatory purposes were found. In humans, the target serum or plasma concentration for analgesia is the same as for antipyresis (20 to 50 mg per L), while targets for anti-inflammatory purposes are generally higher (eg, for treatment of rheumatoid arthritis, target serum concentration is approximately 200 mg per L).9
In 1972, Davis and Westfall10 published pharmacokinetic parameters of SS in pigs. In that study, gelatin capsules of SS were administered orally at three dose levels to swine (n = 4), and the resulting plasma concentrations were reported. Sodium salicylate was also administered intravenously (IV) and the resulting plasma concentrations, half life, and volume of distribution were reported.10 While Davis and Westfall’s study provided important IV pharmacokinetic parameters, plasma concentrations of salicylate after administration of ASA or SS through swine water systems have not been reported.
Due to the widespread use of ASA and SS products despite the lack of oral dosage information, there is potential for harm to treated animals. Products are currently marketed in various forms, including powders and liquids. Only liquid products were investigated in this study due to product availability and widespread use, and to eliminate potential variability associated with mixing a dry product. Thus, the aims of this study were to investigate the stability of stock solutions of ASA and SS, and to determine plasma concentrations in swine following water administration of a liquid SS product.
Materials and methods
Trial One
Study design: All ASA products used in this study claimed to have a concentration of 120 mg active ingredient per mL, and all SS products, a concentration of 485.6 mg active ingredient per mL. A representative liquid SS product (Liquid Asp-Rin; AgriLabs, St Joseph, Missouri) and a representative liquid ASA product (Asp-Rin Concentrate; AgriLabs) were selected for the study. A stock solution of each selected product was prepared, placed in a commercial nursery environment for 24 hours, and sampled at 0, 8, 16, and 24 hours. After initial preparation, stock solutions were not remixed during the 24-hour trial. Concentrations of ASA and SS in these samples were quantified against a standard curve using a previously described analytical high pressure liquid chromatography (HPLC) procedure.11
Preparation of stock solutions: The stock solution for each product was prepared assuming a target dose of 10 mg of active ingredient per kg body weight, average water consumption of 11% of body weight per day, body weight of 18 kg, and medication rate of 1 part stock solution to 128 parts drinking water. Specifically, 88.9 mL of the SS product was added to 3.8 L of water and 354.9 mL of the ASA product was added to 3.8 L of water to achieve a stock solution concentration of 11.2 g per L for each product. As prepared, the SS stock solution concentration was approximately 37% of the labeled stock solution concentration (8 oz per gallon of SS product per gallon, or approximately 240 mL per 3.8 L). As prepared, the ASA stock solution concentration was approximately 12 times the labeled stock solution concentration (1 oz per gallon of ASA product, or 30 mL per 3.8 L).
Trial Two
Study animals and housing. Mixed-breed 9-week-old pigs of commercial genotypes, weighing 18.19 ± 2.75 kg (mean ± SD), were used in this trial. Pigs were housed in the commercial nursery used in Trial One. The building was ventilated by negative pressure and contained 40 central pens plus raised pens along the outside walls. Pens were separated by two walkways, had slatted floors, and contained an average of 4.3 m2 floor space per pen. Each pen contained four nipple waters and a six-hole stainless steel self feeder, and housed an average of 16.4 pigs (range, 11 to 19 pigs per pen). The room was segregated by gender, and only animals in the gilt half of the room were used in the study.
Biosecurity on this site was good (eg, gated entrance, shower facilities, locked exterior doors, current visitor log). No clinical signs were present in the animals at the time of the study. Historical data on previous diseases in the herd were unknown. Animal care in this study was approved by the Committee on Animal Care at Iowa State University.
Selection of treatment product. It was assumed that an 18-kg pig consumes water at the rate of 11% of its body weight per day. If the ASA product is metered into the drinking water at a rate 1:128 and ASA has a water solubility of 3.33 g per L,12 then the highest achievable dose of ASA would be 2.98 mg per kg. The water solubility of SS is 1111.1 g per L.12 Under the given assumptions, the highest achievable dose of SS would be 994.1 mg per kg. Therefore, as a higher dose could potentially be achieved, the SS product was selected for use in Trial Two.
Treatment groups. Fifteen pens were divided into five groups (three pens per treatment group) so that each treatment group received a different dose of the SS product (Liquid Asp-Rin) or no treatment (negative controls). Stock solutions for the five treatment groups were calculated to achieve the following daily doses of SS (based on body weight): 2.22 mg per kg (T1); 4.45 mg per kg (T2); 8.89 mg per kg (T3); and 17.78 mg per kg (T4). The control group (T5) received nonmedicated water.
Stock solution preparation. Concentrations of stock solutions were calculated assuming an SS product with an active ingredient concentration of 485.6 mg per mL, and medication rate of 1 part stock solution to 128 parts drinking water. Stock solutions were prepared every 24 hours for each of the four treated groups. The following stock solution SS concentrations were prepared: 2268 mg per L (T1); 4913 mg per L (T2); 9827 mg per L (T3); and 19,654 mg per L (T4).
Modifications to water delivery system. Treatment and control pens from which animals were sampled (one pen per group of three pens) were plumbed individually with new 1/2-inch internal diameter PVC pipe, 5/8-inch garden hose, 1/2-inch nipple waterers, and 1/2-inch galvanized pipes from the nipples to the fresh water source. Pens were medicated in groups of three to ensure a sufficient flow of water through the medicator; thus, one water medicator (HN55 Chemilizer Medicator; Chemilizer, Largo, Florida) and one water meter (1-inch C-700 Kent Water Meter; Elster AMCO Water, Ocala, Florida) were installed per treatment group. The control pens were plumbed with a water meter only.
Study design. Before the trial began, a sample was collected from the main water supply to the nursery room and tested for coliforms, nitrates-nitrites, and sulfates by a certified water-testing facility and for copper and iron by the Iowa State University Veterinary Diagnostic Laboratory (Ames, Iowa). The pH of the sample was measured with a digital pH meter (PHB-320; Omega Engineering, Stamford, Connecticut). Drinking water for groups T1, T2, T3, and T4 was medicated continuously for 72 hours using stock solutions prepared daily. Samples for assay of salicylate were collected from each stock solution at 0, 24, and 48 hours after preparation. Water volume (in gallons) and specific time of the reading were recorded twice daily throughout the trial for each of the five water meters. One pen per treatment group was selected for collection of water samples from the water nipples at 0, 24, and 48 hours to assay for salicylate (ie, five of the 15 pens were sampled). Water was allowed to flow for 1 minute from two water nipples per pen, then a 9-mL sterile blood vial was filled from each nipple.
Prior to initiation of treatment, a convenience sample of 10 pigs was selected from each of the five sampled pens. Selected pigs were ear-tagged and blood samples were collected for determination of plasma salicylate concentration before initiation of treatment (time 0) and 24, 60, and 72 hours later. At 72 hours, blood samples were collected from all pigs in the trial for determination of hematocrit, and all pigs were then weighed on a digital scale (Salter Electro Samson Hanging Scale; Salter Brecknell Weighing Products, Fairmont, Minnesota).
Assays for salicylate. Water samples were tested for salicylate as described for Trial One. Plasma samples were extracted for salicylate according to the sample preparation procedure of Abu-Qare and Abou-Doniz13 and quantified against a matrix curve of 100, 500, 1000, and 5000 ppb using the analytical HPLC procedure of McMahon and Kelly.11
Statistical analysis. Descriptive statistics of the plasma concentrations of salicylate for each treatment group (including the mean, SD, maximum, and minimum) are reported. The statistical program JMP Statistical Discovery 6.0.0 (SAS, Cary, North Carolina) was used to calculate variables. No statistical comparison of variables was performed. No statistical analysis was performed on the data in this trial, as only one treatment group for each SS stock solution concentration was measured at each time point.
Results
Trial One
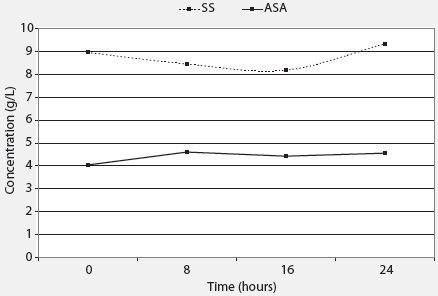
The ASA stock solution crystallized out of solution while the SS product did not. The SS product retained more active ingredient in solution than the ASA product (Figure 1). Concentrations of the stock solutions over the 24-hour study period ranged from 4.04 to 4.61 g per L for the ASA product and from 8.19 to 9.34 g per L for the SS product.
| Figure 1: Changes in concentrations of acetylsalicylic
acid (ASA) and sodium salicylate (SS) in stock solutions exposed to nursery
conditions for 24 hours after preparation (Trial One). The ASA solution
was prepared by adding 354.9 mL of a liquid ASA product to 3.8 L of
water and the SS solution was prepared by adding 88.9 mL of a liquid SS
product to 3.8 L of water to achieve a stock solution concentration of
11.2 g per L for each product. Samples collected at 0, 8, 16, and 24 hours
were quantified against a standard curve using analytical high pressure
liquid chromatography.
|
Trial Two
The coliform bacterial count in the water sample collected from the nursery site before the study began was < 1 colony forming unit (CFU) per 100 mL total (maximum contaminant level [MCL] allowable by the Environmental Protection Agency = 1 per 100 mL). Sulfate concentration was 78 mg per L (MCL = 250 mg per L); nitrate-nitrite concentration was < 0.5 mg per L (MCL = 10 mg per L); and copper and iron levels were < 0.1 mg per L and 0.6 mg per L, respectively. The pH of the water sample was 6.305.
The concentration of SS in the stock solutions at each time point, determined by HPLC analysis, the average concentrations over the 72-hour trial, and the calculated target concentrations for each treatment group are shown in Table 1.
Table 1: Mean concentrations of sodium salicylate in stock solutions used in water medicators for nursery pigs (Trial Two)*
* Drinking water was medicated continuously for 72 hours using stock solutions prepared daily, except in group T5 (negative controls). Treatment groups were housed in three pens per group (11 to 19 pigs per pen; approximately 4.4 m2 of floor space per pen), with four water nipples per pen and one water medicator serving each group. † Salicylate concentration determined by high pressure liquid chromatography. ‡ Average concentration across the 72-hour study. |
||||||||||||||||||||||||||||||||||||||||||||
The concentrations of SS in the water nipple samples at each time point, determined by HPLC analysis, the average concentrations over the 72-hour trial, and the calculated target concentrations for each treatment group are shown in Table 2.
Table 2: Mean concentrations of sodium salicylate in samples of drinking water from water nipples in a system supplied by water medicators (Trial Two)*
* Water system and treatment groups described in Table 1. † Salicylate concentrations determined by high pressure liquid chromatography. ‡ Average concentration across the 72-hour study. |
||||||||||||||||||||||||||||||||||||||||||
Plasma salicylate concentrations for all treatment groups during the trial are shown in Table 3.
Table 3: Mean plasma concentrations of salicylate in groups of nursery pigs treated with a liquid sodium salicylate product in the drinking water for 72 hours (Trial Two)*
* Water system and treatment groups described in Table 1. Concentrations of sodium salicylate in the stock solutions and in drinking water samples from the water nipples are shown in Tables 1 and 2, respectively. |
|||||||||||||||||||||||||||||||||||
Mean body weights (± SD) for the treatment groups at 72 hours
were 18.72 ± 3.63 kg (T1); 16.98 ± 1.80 kg (T2);
17.77 ± 1.88 kg (T3); 17.79 ± 3.49 kg (T4); and 19.73 ± 2.31 kg
(T5). Mean hematocrits (± SD) for the treatment groups at 72 hours
were 0.28 ± 0.027 L per L (T1); 0.27 ± 0.025 L per L (T2); 0.27
± 0.023 L per L (T3); 0.25 ± 0.026 L per L (T4); and 0.28 ±
0.031 L per L (T5). Average hematocrits for all pigs was 0.27 L per
L (reference range for swine,14-16 0.28 to 0.46 L per
L).
Discussion
Under conditions in the nursery barn, the ASA stock solution, prepared at a concentration of 11.2 g per L, crystallized out of solution, while the SS product remained in solution. This agrees with published solubility data, ie, the solubility of ASA in water is 3.33 g per L while the solubility of SS in water is 1111.1 g per L.12
Solubility, the amount of a substance that will dissolve in a given amount of another substance,17 depends on the chemical nature of the compound as well as on temperature and pressure. In Trial One, both compounds were tested under commercial nursery conditions. To simulate normal production temperatures, pressures, and mixing conditions, solutions were not remixed during the 24-hour period. At all times, stock solutions contained numerically less active ingredient of the ASA product than of the SS product. Since even a sample taken directly after mixing shows that stock solutions contained less of the ASA product, it is clear that a medicator would take up more SS product than ASA product. Under the conditions of this study, the highest achievable dose of ASA in swine would be 3.1 mg per kg per day. This dose is considerably less then that recommended for relief of pain in swine, which is 48 mg per kg per day for ASA administered in drinking water.2
The results of the water-quality testing were acceptable for total coliform bacteria and well below the MCLs set by the Environmental Protection Agency for sulfate and nitrate-nitrite. The pH and copper and iron levels were not compared to levels at other production systems within the same geographic area, but are provided as a reference to increase the external validity of this study. These covariates must be taken into account when extrapolating the plasma concentration data from this study to other production situations.
As water consumption is difficult to measure accurately under field conditions, the second trial could not accurately measure individual pig water intake, and hematocrit was tested at the 72-hour sampling period to ensure that there was no clinically important difference in hydration status among the groups. The average hematocrit for all animals was 0.27 L per L (range = 0.22 to 0.32 L per L). The reported “normal” range for swine, 0.28 to 0.46 L per L,14-16 was compiled from available information, including laboratory animal recommendations,14 miniature swine data,15 and slaughter-line blood collection data.16 Readers should note that hematocrit reference ranges for swine are not commonly used. The available references were included to provide a comparison for this study, even though the reference populations varied from the population in this trial. In future studies, hematocrit could be tested at each data collection point, or water disappearance could be monitored throughout the trial to assess the effect decreased water consumption might have on plasma salicylate concentrations.
Plots of plasma concentrations over time after water administration of known quantities of SS are needed to determine the bioavailability of the drug. Bioavailability is calculated by comparing the area under the plasma concentration curve (AUC) to the AUC determined after IV administration in the same study population. The sampling intervals in this study were not sufficient to determine an accurate AUC, and an IV comparison study was not conducted. The data presented in this study will provide a reference for achievable plasma concentrations after administration of an SS product via a water medication system.
The data presented in this study confirm that nursery pigs absorb SS administered in the drinking water. The reason for the decrease in plasma concentrations in the 60-hour samples for all groups is unknown. Samples collected from the water nipples indicated that the amount of SS presented to the pigs was variable, especially at the 24-hour water sample. Daily water-meter readings and stock solution disappearance confirmed water movement through the system at the targeted dosing rate of 1 part stock solution to 128 parts water, and no leaks were observed. However, if the water had been accidentally turned off and back on between sampling periods, the pigs might have compensated by drinking more water, which would result in increased plasma concentrations in the 24-hour samples. The water meter would not show fluctuations in water disappearance, as only the total amount of water through the system was recorded. Although accidental shut-off was possible, it was not noted by the researchers. Actual stock solution concentrations correlated well with the calculated target concentration. However, there was significant variation in instantaneous concentration of salicylate at the water nipple. These fluctuations in SS concentration in the water at any specific time, coupled with variations in the frequency and volume of consumption events between pigs, may introduce significant variation in individual pig dose and subsequent response to treatment. The cyclic, mechanical action of the medicator may have been responsible for the variation in concentration measured at the nipple and the measured discrepancy between the actual and calculated water concentrations, especially in T3 at the 60-hour time point. Larger, thoroughly mixed samples from water nipples should be collected in future studies. Additional evaluation of the variation of water dosing over time is warranted, using a wide spectrum of medication devices employed in pig barns. The reason for the decrease in serum plasma salicylate concentrations for T3 at the 60-hour sampling point is unknown with any certainty, although it correlates with the decrease in concentration of salicylate in the water-nipple samples at this time point.
Implications
- Major differences in the solubilities of ASA and SS products have an impact on product choice, depending on the target dose in the pig.
- Sodium salicylate administered orally through a water-medication system is absorbed and reaches measurable plasma concentrations.
- The consistency of SS dosing using in-line water medicators may be highly variable.
Acknowledgements
This trial was made possible through a grant from the National Pork Board. Special thanks to Hans Coetzee, BVSc, PhD; Elizabeth Fara (technical assistance); and Marianne Kirkendall (technical assistance).
References
1. Davis LE. Clinical pharmacology of salicylates. JAVMA. 1980;176:65–66.
2. Langston C, Apley MD, Boothe DM, Clark TP, Davidson GF, Dowling P, Kemp DT, Papich MG, Riddell MG, Riviere JE, Tubbs RC, Wilcke JR. USP Veterinary Pharmaceutical Information Monographs – Anti-inflammatories. J Vet Pharmacol Ther. 2004;27(S1):4–14.
3. Damian P. Extra label use of nonsteroidal anti-inflammatory drugs. JAVMA. 1997;211:860–861.
4. Kun-Yu Wu K. Biochemical pharmacology of nonsteroidal anti-inflammatory drugs. Biochem Pharmacol. 1998;55:543–547.
5. Jenkins WL. Pharmacological aspects of analgesic drugs in animals: An overview. JAVMA. 1987;191:1231–1240.
6. Vane J, Botting R. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3A):2S–8S.
7. Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002;447:1–9.
8. Plumb DC. Aspirin. Veterinary Drug Handbook. 3rd ed. Ames, Iowa: Iowa State University Press; 1999:56–60.
9. Adams RH. Veterinary Pharmacology and Therapeutics. 8th ed. Ames, Iowa: Iowa State University Press; 2001:439.
10. Davis LE, Westfall BA. Species differences in biotransformation and excretion of salicylate. Am J Vet Res. 1972;33:1253–1262.
11. McMahon GP, Kelly MT. Determination of aspirin and salicylic acid in human plasma by column-switching chromatography using on-line solid phase extraction. Anal Chem. 1998;70:409–414.
12. Budavari S, O’Neil MJ, Smith A, Heckelman PE, eds. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 11th ed. Rahway, New Jersey: Merck and Company Inc; 1989:1367–1368.
13. Abu-Qare AW, Abou-Doniz MB. A validated HPLC method for the determination of pyridostigmine bromide, acetaminophen, acetylsalicylic acid and caffeine in rat plasma and urine. J Pharm Biomed Anal. 2001;26:939–947.
14. LabDiet. Technical information. Biological values. Laboratory swine. Available at: http://labdiet.com/swine-biological.htm. Accessed 9 Jan 2007.
15. Swindle MM, Smith AC, Laber KL, Goodrich JA, Bingel SA. Biology and Medicine of Swine. In: Reuter JD, Suckow MA, eds. Laboratory Animal Medicine and Management. Ithaca, New York: International Veterinary Information Service. 2003. Available at: http://www.ivis.org/advances/Reuter/swiondle/Chapter_frm.asp?LA=1. Accessed 9 Jan 2007.
16. Odink J, Smeets JFM, Visser IJR, Sandman H, Snijders JMA. Hematological and clinicochemical profiles of healthy swine and swine with inflammatory processes. J Anim Sci. 1990;68:163–170.
17. Miriam-Webster Online. Definition of solubility. Available at: http:// www.webster.com. Accessed 9 Mar 2007.