Anil SS, Anil L, Deen J, et al. Factors associated with claw lesions in gestating sows. J Swine Health Prod. 2007;15(2):78–83
| Original research | Peer reviewed |
Cite as: Anil SS, Anil L, Deen J, et al. Factors associated with claw lesions in gestating sows. J Swine Health Prod. 2007;15(2):78–83.
Also available as a PDF.
SummaryObjective: To characterize claw lesions and to analyze the factors associated with types of claw lesions in sows. Methods: Claw lesions were scored for 184 sows in farrowing stalls on day 110 of gestation, and associations with body condition (body weight and backfat thickness on day 109 of gestation), parity, gestation housing system, and lameness were analyzed using logistic regression models. Results: At least one lesion was present on at least one claw on the wall and the heel areas of 88.6% and 86.4% of sows, respectively, with more severe lesions on forelimbs and on lateral claws. The likelihood of wall lesions in any claw increased with body weight on day 109 of gestation. For any claw, the likelihood of heel lesions increased and the likelihood of overgrown heels decreased with higher backfat. Non-lame sows were less likely than lame sows to have white-line lesions on any claw. Sows of parity ≤ 5 were more likely to have white-line lesions in any claw than sows of parity > 5. Sows housed in pens with electronic sow feeders (ESF) were more likely than stall-housed sows to have all types of lesions in any claw. Implications: Under the conditions of this study, lameness and younger parity were associated with white-line lesions. Use of ESFs was associated with more severe lesions. Measures to minimize claw lesions in sows are needed to reduce the incidence of lameness and probably removal rates, especially for younger parities. | ResumenObjetivo: Describir lesiones de pezuña y analizar los factores asociados con los tipos de lesiones de pezuña en hembras. Métodos: Se calificaron las lesiones de pezuña de 184 hembras en jaulas de maternidad desde en el día 110 de la gestación, y se analizaron asociaciones con la condición corporal (peso corporal y grosor de la grasa dorsal en el día 109 de la gestación), paridad, sistema de alojamiento de gestación, y cojera, utilizando modelos de regresión logística. Resultados: Al menos una lesión estuvo presente en por lo menos en una pezuña en las áreas de pared y talón (88.6% y 86.4% de hembras, respectivamente), con lesiones más severas en las extremidades delanteras y en las pezuñas laterales. La probabilidad de lesiones de pared en cualquier pezuña aumentó con el peso corporal en el día 109 de la gestación. Para cualquier pezuña, la probabilidad de lesiones de talón aumentó y la probabilidad de talones muy crecidos disminuyó con mayor espesor de grasa dorsal. Las hembras sin cojera tuvieron menos probabilidad que las hembras con cojera de tener lesiones de línea blanca en cualquier pezuña. Las hembras de paridad ≤ 5 tuvieron mayor probabilidad de tener lesiones de línea blanca en cualquier pezuña que las hembras de paridad > 5. Las hembras alojadas en corrales comederos electrónicos (ESF por sus siglas en inglés) tuvieron mayor probabilidad que las hembras alojadas en jaulas de tener todo tipo de lesión de pezuña en cualquier pezuña. Implicaciones: Bajo las condiciones de este estudio, la cojera y la paridad más joven estuvieron relacionadas con lesiones de línea blanca. La utilización de los ESF estuvo relacionada con lesiones más severas. Las medidas para minimizar las lesiones de pezuña en hembras son necesarias para reducir la incidencia de cojera y probablemente el porcentaje de desecho, especialmente en paridades jóvenes. | ResuméObjectif: Caractériser les lésions aux onglons et analyser les facteurs associés aux types de lésions aux onglons chez les truies. Méthodes: Les lésions aux onglons de 184 truies logées dans des cages de mise-bas ont été cotées au jour 110 de la gestation, et des associations avec l’état de chair (poids corporel et épaisseur du gras dorsal au jour 109 de gestation), la parité, le type de logement durant la gestation, et la boiterie ont été analysées à l’aide de modèles de régression logistique. Résultats: Au moins une lésion était présente sur au moins un onglon dans la région de la paroi ou du talon (respectivement 88.6% et 86.4%), avec des lésions plus sévères sur les pattes avant et sur les onglons latéraux. La probabilité d’une lésion sur la paroi de n’importe quel onglon augmentait avec le poids corporel au jour 109 de gestation. Pour n’importe quel onglon, la probabilité de lésions au talon augmenta et la probabilité de croissance exagérée au talon diminua avec une plus grande épaisseur de gras dorsal. Les truies sans boiterie étaient moins susceptibles que les truies avec boiterie d’avoir des lésions à la ligne blanche sur n’importe quel onglon. Les truies avec ≤ 5 parités étaient plus susceptibles d’avoir des lésions à la ligne blanche sur n’importe quel onglon que les truies avec > 5 parités. Les truies logées dans des parcs avec des distributeurs d’aliment électronique (ESF) étaient plus susceptibles que les truies logées dans des enclos (stalles) d’avoir tous les types de lésions sur n’importe quel onglon. Implications: Dans les conditions de la présente étude, les boiteries et une parité plus jeune étaient associées avec des lésions à la ligne blanche. L’utilisation d’ESF était associée avec des lésions plus sévères. Des mesures pour minimiser les lésions aux onglons des truies sont nécessaires pour réduire l’incidence de boiterie et probablement le taux de réforme, surtout pour les truies de basses parités. |
Keywords: swine, claw
lesions, lameness, parity, housing system
Search the AASV web site
for pages with similar keywords.
Received: April
5, 2006
Accepted: July
31, 2006
Hoof lesions are very common in pigs and are an important underlying cause of lameness.1 Although sows with mild lesions may show no overt signs of pain, severe hoof lesions may cause lameness. In addition, lameness may result when hoof lesions permit entry of infections that spread upwards, affecting joints. Lameness is a welfare concern and a major reason for early removal of sows from breeding herds,2 causing producers considerable economic loss. Housing conditions and management practices may be associated with development of foot lesions.3
Specific factors associated with hoof lesions in pigs include the interaction between the floor surface and the horn of the claw,4 physical properties of the floor,5 and nutrition, especially related to dietary biotin levels.4 Studies on hoof lesions in pigs are relatively rare. It is important to characterize and understand the factors associated with claw lesions to minimize the incidence of such lesions and to reduce removal of sows for lameness. The objectives of this study were to characterize claw lesions and to identify factors associated with different types of claw lesions in sows in a breeding herd in Minnesota.
Materials and methods
Animals, housing, and feeding
The study was conducted at the University of Minnesota, Southern Research and Outreach Center, Waseca, Minnesota. All protocols were approved by the institutional Animal Care and Use Committee of the University of Minnesota. One hundred and eighty-four sows (Genetically Advanced Pigs, GAP Genetics, Winnipeg, Manitoba, Canada) of parities 0 to 8 and weighing 166 to 337 kg at 109 days of gestation were included in the study. Sows were housed either in pens (12.75 m × 6.75 m) with one electronic sow feeder (ESF) per pen (TEAM electronic sow feeder; Osborne Industries, Osborne, Kansas) or in stalls (Crystal Spring Hog Equipment Ltd, St Agathe, Manitoba, Canada; length 200 cm, width 60 cm, height 97 cm). Pens and stalls had fully slatted flooring (solid portion 12.7 cm wide and 12.7 cm deep; slots 2.54 cm wide). Among the study sows, 102 had been housed in group pens during gestation throughout their life in the herd, and 82 had been housed in stalls during gestation. Twenty-one parity-one sows, 32 parity-two sows, 26 sows of parities 3 to 5, and 23 sows of parities > 5 were housed in pens, and 16 parity-one sows, 27 parity-two sows, 18 parity of parities 3 to 5, and 21 sows of parities > 5 were housed in stalls. Distributions of parities in group-housed and stall-housed sows did not differ (chi-square test; P > .05). Until day 109 of gestation, sows were fed 2.2 to 3.0 kg of feed daily (crude protein content 15%) on the basis of body weight and backfat at weaning. On gestation day 109, sows were weighed on a digital Ag Alliance scale (Altoona, Iowa) and backfat was measured at the last rib (5.08 cm from the midline of the back on both left and right sides) with a Lean-Meater ultrasound unit (Renco, Minneapolis, Minnesota). Mean backfat thickness for group-housed sows (19.76 mm; SE, 0.38 mm) and stall-housed sows (18.55 mm; SE, 0.60 mm) did not differ (two-sample t-test; P > .05). Sows were then washed and moved into farrowing stalls with cast-iron flooring (214 cm × 66 cm, excluding a creep area for the piglets). Sows in the farrowing stalls were offered 3 kg of feed per day until farrowing, and ad libitum feed during lactation. Sows were weaned at an average of 18.8 days of lactation (SE, 0.14 day).
Claw lesions and scoring system
Claws were individually examined for lesions on day 110 of gestation when sows were in the farrowing stalls. Lesions included erosions, cracks, and overgrowths. The horny wall and the volar (plantar) surface of the hoof were examined. The medial and lateral claws of each foot were examined for lesions on a severity scale6 of 0 (no lesions noted) to 4 (severe) (Table 1). Areas on the claw were classified as wall (composed of hard keratinized epidermis), heel (soft keratinized epidermis on the ventral aspect of the claw towards the posterior end, including overgrown heel), sole (hard keratinized epidermis anterior to the heel on the ventral aspect of the claw), junction between heel and sole, white line (junction between sole and wall), and toe (anterior part of the sole). The final score on each area was obtained by multiplying the number of lesions by the severity of these lesions. The total score for each claw was obtained by summing the scores for different areas of the claw. Total score on each foot was obtained by summing scores for different areas of the two claws. Scores on all areas of all feet were summed to obtain total claw-lesion scores.
Table 1: Scoring method6 for claw lesions in a study examining 184 sows at day 110 of gestation
|
Sows were made to walk a short distance within the farrowing room and were observed for difficulty in bearing weight on one or more limbs. Sows were categorized as either lame or non-lame. The same individual scored lesions and evaluated lameness in all sows, and was unaware of the gestation housing system at the time of scoring.
Statistical analysis
Median and range were used to describe the data collected on lesions. All analyses were performed using SAS software (Statistical Analysis System, version 9.1; SAS Institute Inc, Cary, North Carolina). The proportions of sows with and without lesions on different areas in the lateral and medial claws of front and hind limbs were compared using one-sample and two-sample proportion tests. Separate logistic regression models (Proc Logistic, SAS) were fitted (Wald statistic) to analyze the association of lameness, parity, gestation housing system, and body condition (backfat and body weight) at 109 days of gestation with the presence or absence of lesions on different areas of the claw. For analysis, parity of the sows was categorized as P1 (parity 1), P2 (parity 2), P3-5 (parities 3 to 5), and P > 5 (parities > 5). Gestation housing system was categorized as pens with ESF or stalls, and lameness was categorized as lame or non-lame. A P value of < .05 was considered significant in all analyses.
Results
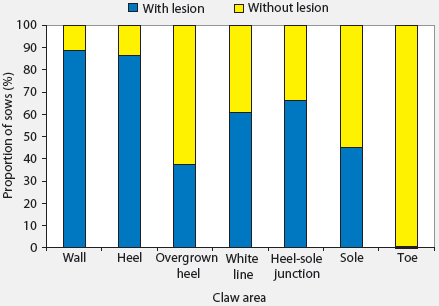
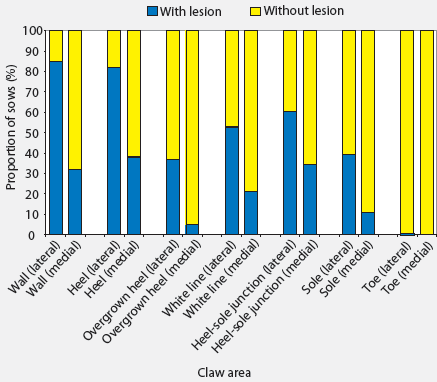
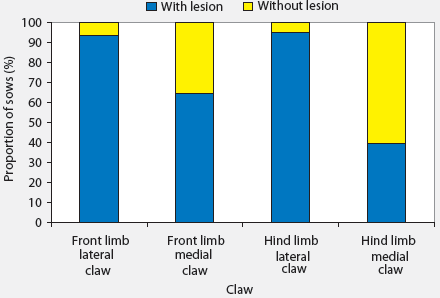
Median and range of lesion scores on different areas of claws are presented in Table 2. Only seven of the 184 sows had no lesions on any claw area, and these sows were all housed in stalls during gestation. More severe lesions were seen on walls and heels. Among the 184 sows examined and considering all claws, 88.6% had at least one wall lesion and 86.4% had at least one heel lesion (Figure 1). Other areas where lesions were common were the junction between heel and sole (66.3% of sows affected) and the white line (60.9% of sows affected). Toe lesions were relatively uncommon (0.5%). The proportions of sows with lesions were greater than the proportions without lesions (P < .05) in all claw areas except the sole. Lesions were more severe on forelimbs than on hind limbs (Table 2), and the proportions of sows with lesions on different areas of the lateral claws were greater (P < .05 for all except toe lesions) than the proportions of sows with lesions on different areas of the medial claws (Figure 2). The proportions of sows with lesions on the lateral claws were greater (P < .05) for both front and hind limbs (Figure 3). The proportions of sows with claw lesions were greater (P < .05) among sows housed during gestation in group pens with ESF than in those housed in stalls (Table 3), with the exception of toe lesions. Among the sows with claw lesions, 60.7% with wall lesions, 60.0% with heel lesions, 63.4% with white line lesions, 67.0% with lesions at heel-sole junction, 57.8% with sole lesions, and 75.4% with overgrown heels were housed in group pens during gestation. The proportion of lesions with severity > 2 was numerically larger in the wall and heel areas than in other areas of the foot, but this proportion was not statistically analyzed.
Table 2: Median and range of lesion scores* on different areas of claws across all limbs in 184 sows at day 110 of gestation
* Scores defined in Table 1. The final score for each area = number of lesions × lesion score. Total score for each claw = sum of scores for different areas of the claw. Total score per foot = sum of scores for both claws. Total claw lesions = sum of scores for all four feet. |
|||||||||||||||||||||||||||||||||||||||||||||||||
| Figure 1: Proportions of 184 sows with and without
lesions on different claw areas. The proportions of sows with lesions were
greater than the proportions without lesions for all claw areas except
the sole (P < .05; one-sample proportion test). Toe lesions were
relatively uncommon and were not compared.
|
| Figure 2: Proportions of 184 sows with and without
lesions on different areas of lateral and medial claws. The proportions
of sows with lesions on lateral claws were greater than the proportions
with lesions on medial claws for all claw areas except the toe (P < .05;
two-sample proportion test).
|
| Figure 3: Proportions of 184 sows with and without
lesions on lateral and medial claws of front and hind limbs. Differences
were significant for proportions of sows with and without lesions within
each limb and claw (P < .05; one-sample proportion test) and
for proportions with lesions in medial and lateral claws within each limb
(P < .05; two-sample proportion test).
|
Table 3: Proportions of sows with hoof lesions according to type of gestation housing*
* Sows were housed in pens with electronic sow feeders (n = 102) or in gestation stalls (n = 82). Lesions were assessed on day 110 of gestation. † For all claw areas except the toe, proportions of sows with and without lesions differed for pen housing and stall housing (P < .05; two-sample proportion test). |
|||||||||||||||||||||||||||||||||||||||||||||||
The odds ratios and confidence intervals indicating the association of lesions on different areas of the claw with body condition, lameness, parity, and gestation housing system are presented in Table 4. Since the overall prevalence of toe lesions was low, they were not included in the regression analysis. The likelihood of wall lesions increased 3% with an increase of 1 kg of body weight on day 109 of gestation. Sows with higher backfat on day 109 of gestation had a 15% greater likelihood of having heel lesions (P < .05) and a 12% lesser likelihood of having overgrown heels (P < .05). Non-lame sows had a 62% lesser likelihood of having white-line lesions (P < .05) than lame sows. The likelihood of having white-line lesions was 5.5, 4.5, and 2.9 times greater in sows of parities P1, P2, and P3 to P5, respectively, than in sows of parity > 5 (P < .05). The likelihood of having all types of claw lesions (overgrown heel and lesions on wall, heel, white line, junction between sole and heel, and sole) was greater in sows housed in pens with ESF (P < .05). The likelihood of having wall lesions, heel lesions, overgrown heel, white-line lesions, and lesions at the junction between sole and heel were approximately 10.0, 3.5, 5.0, 2.0, and 5.0 times greater, respectively, in sows housed in pens with ESF during gestation than in sows housed in stalls.
Table 4: Odds ratios (OR) and confidence intervals (CI) for association of body condition (indicated by P2 backfat thickness and body weight at gestation day 109), lameness,* parity, and gestation housing system† with presence or absence of lesions on different claw areas in 184 sows
* Lame or not lame. † Sows had either been group-housed in pens with electronic feeders during each gestation (n = 102), or housed in gestation stalls during each gestation (n = 82). All sows were moved into farrowing crates the day before they were examined for lameness and scored for foot lesions. abc Level of significance (Wald statistic): a, P < .05; b, P < .01, and c, P > .05 (variables did not differ). Sole lesions did not differ among variables (P > .05). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
The smaller number of sows without any claw lesions may be linked to the intensive selection and management in today’s swine industry that has made pigs grow rapidly to a large body weight. It has been suggested that this rapid growth affects the soundness of legs and feet.3 The findings of the present study are thus in agreement with a previous report6 that more than 96% of loose-housed sows and 80% of confined sows had at least one lesion on the lateral hind claws. The most prevalent lesions reported in that study6 were hoof-wall cracks, heel lesions, cracks in the white line, and overgrown heels. In the present study, the majority of sows had lesions on the wall, heel, and white line. In a recent study,7 it was reported that hyperkeratinization of soft heel tissue was observed in 74% to 84% of sows autopsied. Other common claw lesions observed were cracks in the hoof wall (44% to 49%), cracks in the sole (75% to 77%), and cracks in the white line (63% to 65%).7
The weight distribution of sows may be an important factor determining development of lesions on different claws and different limbs.3 Lesions may not develop equally on all claws, as observed in the present study. A previous study4 also indicated more lesions on fore and hind lateral claws than fore and hind medial claws, as in the present study. Hoof lesions may be more common in lateral claws because they have a greater weight-bearing surface than medial claws.8 More than 75% of the weight of the pig is born by the lateral digits, and 80% of injuries affect these digits.9 The strength of different parts of the foot may also vary, and junctions between hard and soft areas may be more susceptible to injuries.3 In the lateral digit, maximum weight is born by the heel bulb, followed by the junction between the heel bulb and the abaxial hoof wall, whereas the tip of the toe is the greatest weight-bearing region in the inner digit.9 In agreement with these reports, greater proportions of sows in this study had lesions at the heel-sole junction and on lateral rather than medial claws.
The floors in the stalls and pens in this study were fully slatted. The greater freedom of movement among sows housed in pens with ESF, and especially the greater activity associated with aggression at mixing and on entry into the feeder, may have been associated with the greater proportion of claw lesions in these sows. Severe lesions (lesion score > 2) on the wall and heel might also be associated with the slatted floor, ie, a foot catching between slats is a source of claw lesions. A higher incidence of claw problems in sows housed on partially slatted concrete floors than in sows on straw-bedding or solid concrete floors has already been reported.10 The space between slats, roughness of the surface, and edge design are crucial factors in deciding the extent of injury.11 When body weight increases, the pressure exerted per unit area of the claw is greater, increasing the chance of injury. This might explain the greater likelihood of wall lesions with an increase in body weight on gestation day 109 and greater likelihood of heel lesions with an increase in backfat thickness on gestation day 109. However, the negative association between backfat thickness and overgrown heel could not be explained.
Foot lesions have long been recognized as a cause of lameness.12 In this study, analysis suggested a link between lameness and white-line lesions. Other claw lesions were not significantly related to lameness. While the wall and sole consist of reinforced tubular horn, the white line is the cemented junction of wall and sole and is an inherent point of weakness.13 In cattle, it has been reported that injuries to the weak white line may easily penetrate the corium and facilitate spread of infection, causing lameness.14 Therefore, white-line lesions are more likely to cause lameness than other types of claw lesions. Although the relationship between claw lesions and lameness is obvious, occurrence of claw lesions is only one factor causing lameness in pigs. It has been reported15 that in cows, lameness may occur in the absence of lesions and that observed lesions may not necessarily cause lameness. The greater likelihood of white-line lesions in lower parities in this study may have occurred because older sows with severe white-line lesions might have already been removed from the herd for lameness or old age, excluding them from analysis.
The amount and type of activity of sows may determine the type and severity of claw lesions, and these two factors varied greatly in gestation stalls and group pens with ESF. The ESF allows only one sow at a time to eat, resulting in a highly competitive situation, especially since the sows were on a restricted diet during gestation. Fighting and aggressive activity at mixing and subsequently at feeding time may increase the likelihood of claw lesions in sows housed in group pens with ESF systems.16 The greater proportion of sows with claw lesions in pens with ESF compared to that in sows housed in stalls may have been due to the greater mobility of sows in the ESF system. Although a greater proportion of sows with claw lesions in pens is partly attributable to the ESF and associated aggression at feeding time, the extent of this effect has not been differentiated in this study.
Measures to minimize the incidence of claw lesions may have to focus on nutrition and management. Biotin supplementation of barley-based diets may reduce claw lesions.4,17 Claw problems have been reported as more common in sows housed on slatted floors than in sows on solid floors with straw bedding, and also when stocking density is higher.18 Rough concrete, worn or uneven slats, and sharp protruding objects increase the risk of lesions. In slatted floors, the void ratio also influences development of claw lesions.9 Stable group structure in group pens may reduce opportunities for aggression and trauma.19 Similarly, minimizing chances of upward infection through claw lesions helps to reduce incidence of lameness.
Implications
- Under the conditions of this study, lameness and younger parity were associated with white-line lesions.
- Sows housed in group pens with ESF are likely to have more severe lesions in all areas of the claw than stall-housed sows.
- Measures to minimize claw lesions would help to reduce incidence of lameness and minimize removal of sows, especially younger parity sows.
References
1. Dewey CE, Friendship RM, Wilson MR. Clinical and postmortem examination of sows culled for lameness. Can Vet J. 1993;34:555–556.
2. Anil SS, Anil L, Deen J. Evaluation of patterns of removal and associations among culling because of lameness and sow productivity traits in swine breeding herds. JAVMA. 2005;226:956–961.
3. Kroneman A, Vellenga L, Van der Wilt FJ, Vermeer HM. Review of health problems in group-housed sows, with special emphasis on lameness. Vet Q. 1993;15:26–29.
4. Simmins PH, Brooks PH. Supplementary biotin for sows: Effect on claw integrity. Vet Rec. 1988;122:431–435.
5. Jensen AH. The effects of environmental factors, floor design and materials on performance and on foot and limb disorders in growing and adult pigs. Proc Pig Vet Soc. 1979;5:85–94.
6. Gjein H, Larssen RB. Housing of pregnant sows in loose and confined systems – a field study. 2. Claw lesions: morphology, prevalence, location and relation to age. Acta Vet Scand. 1995;36:433–442.
7. Kirk RK, Svensmark B, Ellegaard LP, Jensen HE. Locomotive disorders associated with sow mortality in Danish pig herds. J Vet Med A. 2005;52:423–428.
8. Tubbs RC. Lameness in sows: Solving a preventable problem. Vet Med. 1988;83:610–616.
9. Webb NG. Compressive stresses on, and the strength of, the inner and outer digits of pig’s feet and the implications for injury and floor design. J Agric Eng Res. 1984;30:71–80.
*10. Holmgren N, Eliasson-Selling L, Lundeheim N. Claw and leg injuries in group housed dry sows. Proc IPVS. Melbourne, Australia. 2000;352.
11. Boon CR, Wray C. Building design in relation to the control of diseases of intensively housed livestock. J Agric Eng Res. 1989;43:149–161.
12. Penny RHC, Osborne AD, Wright AI. The causes and incidence of lameness in store and adult pigs. Vet Rec. 1963;75:1225–1235.
13. Budras KD, Mülling C, Horowitz H. Rate of keratinization of the wall segment of the hoof and its relation to width and structure of the zona alba (white line) with respect to claw disease in cattle. Am J Vet Res. 1996;57:444–455.
14. Kempson SA, Logue DN. Ultrastructural observations of hoof horn from dairy cows: changes in the white line during the first lactation. Vet Rec. 1993;132:524–527.
*15. Logue DN, Lawson A, Roberts D, Hunter EA. The effect of two different protein sources in the diet upon the incidence and prevalence of lameness in dairy cattle [abstract]. Anim Prod. 1989;48:636.
16. Anil L, Anil SS, Deen J, Baidoo SK, Wheaton JE. Evaluation of welfare indicators, productivity and longevity of sows in pens with electronic sow feeders (ESF) and in stalls. Am J Vet Res. 2005;66:1630–1638.
17. Bryant KL, Kornegay ET, Knight JW, Veit HP, Notter DR. Supplemental biotin for swine. 3. Influence of supplementation to corn- and wheat-based diets on the incidence and severity of toe lesions, hair and skin characteristics and structural soundness of sows housed in confinement during four parities. J Anim Sci. 1985;60:154–162.
18. Jorgensen B. Influence of floor type and stocking density on leg weakness, osteochondrosis and claw disorders in slaughter pigs. Anim Sci. 2003;77:439–449.
19. Anil L, Anil SS, Deen J, Baidoo SK, Walker RD. Effect of group size and structure on the welfare and performance of pregnant sows in pens with electronic sow feeders. Can J Vet Res. 2006;70:128–136.
* Non-refereed references.