Amass SF, Thompson B, Dimmich KM, et al. Impact of downtime on reducing aerobic bacterial counts in cleaned and disinfected trailers. J Swine Health Prod. 2007;15(1):37–41
| Brief communication | Peer reviewed |
Cite as: Amass SF, Thompson B, Dimmich KM, et al. Impact of downtime on reducing aerobic bacterial counts in cleaned and disinfected trailers. J Swine Health Prod. 2007;15(1):37–41.
Also available as a PDF.
SummarySwab samples from upper and lower deck floors of nine swine breeding-stock trailers were collected before, immediately after, 1 day after, and 2 days after washing, disinfecting, and drying the trailers. Time after decontamination procedures (downtime) did not affect median aerobic bacterial counts. | ResumenSe recolectaron muestras de hisopo de los pisos superior e inferior de nueve trailers de transporte de cría porcino antes, inmediatamente después, 1 día después, y 2 días después de lavar, desinfectar, y secar los trailers. El tiempo posterior a los procedimientos de descontaminación (tiempo de descanso) no afectó la mediana del conteo de bacterias aeróbicas. | ResuméDes écouvillonnages du plancher des ponts supérieurs et inférieurs de remorques servant au transport des animaux appartenant à neuf troupeaux d’animaux reproducteurs ont été prélevés avant, immédiatement après, 1 jour après, et 2 jours après lavage, désinfection, et séchage des remorques. La durée de la période d’indisponibilité n’a pas modifié la médiane des comptes bactériens aérobies. |
Keywords: swine, trailer,
disinfection, downtime
Search the AASV web site
for pages with similar keywords.
Received: November
9, 2005
Accepted: March
13, 2006
Contaminated livestock vehicles have been reported as risk factors for transmission of, or sources of infection for, classical swine fever,1 mycoplasmal pneumonia,2 Actinobacillus pleuropneumoniae,2,3 Salmonella,4-6 and Escherichia coli 6 under field conditions. In contrast, transmission of porcine reproductive and respiratory syndrome virus (PRRSV) by vehicles has been reported only under simulated experimental conditions, ie, sport utility vehicle7 or 1:150 scale models of trailers8,9 intentionally contaminated with cultures of PRRSV.
The above reports1-9 suggest that decontamination of livestock transport vehicles is an important component of an effective biosecurity program. General cleaning, disinfection, and drying protocols for livestock vehicles have been previously published.10-12 Vehicle decontamination techniques specific for PRRSV, Salmonella, and E coli, using both trailers6 and 1:150 scale models of trailers,9,13-16 have also been tested and published.
Some swine operations have polices that restrict the use of transport vehicles for a period of time after decontamination. Such downtime periods may further reduce contamination following disinfection and drying. Prior to the use of thermo-assisted drying and decontamination (TADD)14 at Pig Improvement Company (PIC) USA, downtimes were in place to allow for natural drying. However, complete drying frequently did not occur during the colder months of the year. Scientific evidence demonstrating that downtime for trailers following decontamination reduces the number of infectious organisms is lacking in the literature. This study was designed to test whether a further reduction in bacterial counts would result if policy required swine transport trailers to have a 1- to 2-day downtime after washing, disinfection, and drying before being reused to transport swine. Because bacteriological swab cultures are considered a practical method for estimating disinfectant efficacy in the field,17 aerobic bacterial counts were used as a marker to determine the effectiveness of downtime.
Materials and methods
Protocol for cleaning and disinfecting trailers
Six 14.6-meter and three 11-meter, 5-year-old to 9-year-old, double-decker trailers (M.H. EBY, Inc, Blue Ball, Pennsylvania) owned by PIC USA were used in this trial. All sampling of trailers and the washing, disinfection, and drying of trailers was performed by PIC USA personnel in Franklin, Kentucky. Trailers were decontaminated according to company protocol. Briefly, manure, bedding, and debris were scraped from the cargo area before the truck entered the wash bay. In the wash bay, both decks of the cargo area were rinsed with a garden hose (water temperature 82.2°C) until free of manure and shavings. Detergent (Magnum 600; Niagara National Corp, Atlanta, Georgia) was applied, at 15 mL per L according to label directions, to all interior surfaces of pig space, and then to the exterior of the vehicle. The exterior and then the interior of the vehicle were pressure washed at 2000 psi at a rate of 4 gallons (15 L) of water per minute. A combination glutaraldehyde-quaternary ammonium disinfectant (Synergize cleaner-disinfectant; Preserve International, Zephyr Cove, Nevada) was applied to all surfaces with dispensers set at 4 mL per L according to label directions. Trucks were dried by parking on an incline. Trailers were drained for 15 minutes to 1 hour then dried with a minimum of 2.0 to 2.5 million BTU using grain-dryer units. Each trailer was dried for 15 to 45 minutes until the entire trailer was visibly dry. The longer the drain time, the shorter the dry time. Trailers were parked in a clean area following heat-assisted drying.
Collection and processing of swab samples
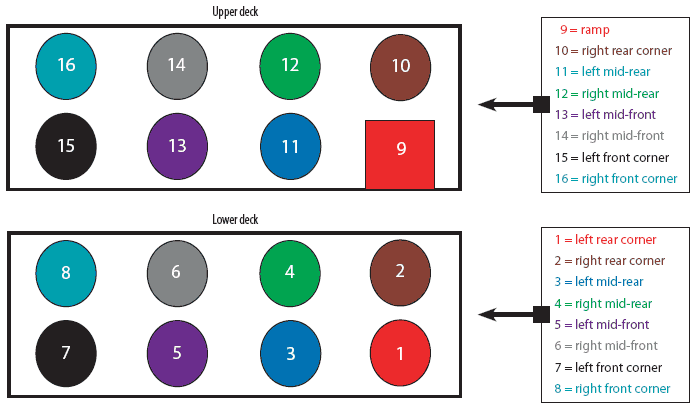
This experiment took place at the end of May 2005. Trailers were sampled by PIC personnel before cleaning (Day -1), immediately after washing, disinfection, and drying (Day 0), 1 day after washing, disinfection, and drying (Day 1), and 2 days after washing, disinfection, and drying (Day 2). Investigators donned dust masks, disposable boots, and gloves during sample collection to prevent contamination of samples. Briefly, 12.84-cm2 swab samples of the designated floor surface were aseptically collected from each trailer using individual sterile metal washers to control sampling area. Swab samples were placed in individual sterile tubes containing 2 mL of sterile chemical broth to inactivate residual disinfectant (D/E Neutralizing Broth; Becton-Dickenson, Franklin Lakes, New Jersey). The swab portion was broken off into the tube of broth and the contents were mixed by hand agitation. A total of six samples from haphazard locations were collected from each trailer before cleaning. Then, a total of 16 samples (eight per deck) were collected from each trailer at Day 0, 1, and 2 (Figure 1). Samples were refrigerated until shipment, and shipped overnight on cold packs to the Purdue University Production Medicine Laboratory, West Lafayette, Indiana. Samples were refrigerated on arrival and processed approximately 72 to 96 hours after collection on a delayed schedule. Prior to dilution and culture, all samples were mixed by hand agitation. A 100-µL aliquot of the original sample was plated directly onto 5% sheep blood agar. Additionally, serial tenfold dilutions were made using sterile D/E Neutralizing Broth, and a 100-µL aliquot of each dilution was plated directly onto 5% sheep blood agar. Samples were incubated at 36.9°C for 24 hours. Colonies of aerobic bacteria were counted. Median, minimum, maximum, and total aerobic bacterial counts were calculated. Morphologically distinct colonies collected at each sampling period were sent to Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa, for identification.
| Figure 1: Location of 16 floor sampling sites in
double-decker swine transport trailers swabbed to determine aerobic bacterial
counts before and after washing and disinfection of the upper and lower
decks of the trailer interior.
|
Calculations and statistical analysis
Average bacterial counts. Average bacterial counts were calculated by summing the total number of bacteria for each trailer at each location and dividing the total by the number of locations sampled at each time period. The average count was calculated for all nine trailers at each sampling period. Average counts were used because the number of locations sampled differed (n = 6 samples for Day -1 and n = 16 samples for Days 0, 1, and 2). The Friedman Test (nonparametric repeated measures ANOVA) was used to compare the median of the average bacterial counts over time, then Dunn’s multiple comparisons test was used to compare median bacterial counts between time periods.
Total aerobic bacterial counts. Total aerobic bacterial counts for each trailer were calculated by summing the total number of bacteria isolated at each of the 16 locations. The total number of bacteria was the number of bacteria isolated from a 205.44-cm2 sampling area (12.84 cm2 per location × 16 locations) for each trailer. Day -1 counts were excluded from these calculations because only six locations were sampled. The Friedman test was used to compare the medians of the total bacterial counts over Day 0, Day 1, and Day 2.
Trailers meeting the recommendation for prophylactic disinfection of animal facilities. Böhm10 recommended 103 colony forming units (CFU) per cm2 as a general target for disinfection of livestock facilities. The number of CFU per cm2 was calculated for each trailer at each time period by summing the total number of bacteria isolated for all sample locations and dividing that number by the total number of cm2 sampled for each time period (six Day -1 locations per trailer = 77.04 cm2; 16 sample locations for Days 0, 1, and 2 = 205.44 cm2 per time period per trailer). The number of trailers that met the criterion for disinfection at each data point were compared using Fisher’s exact test.
Aerobic bacterial counts by trailer.
Day -1, Day 0, Day 1, and Day 2 bacterial counts at each location
were compared by trailer. The Kruskal-Wallis test (nonparametric
ANOVA) was used to compare the median bacterial counts over time,
then Dunn’s multiple comparisons test was used to compare median
bacterial counts between trailers.
Aerobic bacterial counts by location. Day -1 counts were excluded from this analysis because only six locations were sampled and the same six locations were not used for each trailer. Total bacterial counts for each location were calculated by summing the total number of bacteria isolated from each trailer at that location. This number would represent the sum of bacteria isolated from the 12.84-cm2 sampling area of each trailer for each location for each sampling period. The Kruskal-Wallis test (nonparametric ANOVA) was used to compare the median total bacterial counts by location for Days 0, 1, and 2.
A P value of < .05 was considered significant for all statistical tests.
Results
Comparisons of bacterial counts by day
Average aerobic bacterial counts over time. The median average bacterial count for Day -1 was significantly greater than those for Day 1 and Day 2 (Table 1). The median average count for Day -1 was not significantly different from that for Day 0. Differences in median average counts among Days 0, 1, and 2 were not significant.
Table 1: Median, minimum, and maximum average aerobic bacterial counts* for nine swine transport trailers at each sampling period†
* Colony forming units per 12.84 cm2. † Sampling periods were before (Day -1), immediately after (Day 0), 1 day after (Day 1), and 2 days after (Day 2) washing, disinfecting, and drying the trailers. ab Median average bacterial counts with different superscripts were different when compared over time (Friedman test [nonparametric repeated measures ANOVA] followed by Dunn’s multiple comparisons test; P < .01). |
|||||||||||||||||||||||||
Total aerobic bacterial counts over time for Days 0, 1, and 2. The median total bacterial counts were not significantly different for Day 0, Day 1, and Day 2.
Number of trailers meeting the recommended target for disinfection. No trailers met the standard of disinfection (ie, < 103 cfu per cm2) at Day -1 (Table 2). Nine of nine trailers (100%) met this standard of disinfection on Days 0, 1, and 2. Significantly more trailers met this standard of disinfection on Days 0, 1, and 2 compared to Day -1 (Table 2).
Table 2: Colony forming units of aerobic bacteria per cm2 sampled for nine swine transport trailers at each sampling period*
* Sampling periods described in Table 1 ab Number of trailers meeting criteria for disinfection (< 103 cfu per cm2) were different when compared at each time point (Fisher’s exact test; P < .001). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Comparisons of bacterial counts by trailer
Day -1 aerobic bacterial counts by trailer. Trailers 3, 7, and 9 had the lowest median Day -1 bacterial counts (Table 3). Median Day -1 bacterial counts for Trailer 7 were significantly less than for Trailer 1, Trailer 2, and Trailer 8. Median Day -1 bacterial counts for Trailer 3 were significantly less than for Trailer 2, and Trailer 8. Median Day -1 bacterial counts for Trailer 9 were significantly less than for Trailer 8.
Table 3: Median, minimum, and maximum aerobic bacterial counts for nine swine transport trailers at Day -1, with n = 6 sampling locations per trailer*
* Sampling periods are described in Table 1 and sampling locations in Figure 1. † For each trailer, superscripts represent the IDs of other trailers with significantly different median bacterial counts (compared using the Kruskal-Wallis test [nonparametric ANOVA] followed by Dunn’s multiple comparisons test; P < .05). |
||||||||||||||||||||||||||||||||||||||||||||||
Day 0, Day 1, and Day 2 aerobic bacterial counts by trailer. Median Day 0 bacterial counts did not differ among trailers (Table 4). On Day 1, median bacterial counts for Trailer 8 were significantly greater than for Trailer 1, Trailer 2, and Trailer 3. Median Day 2 bacterial counts did not differ among trailers.
Table 4: Median, minimum (min), and maximum (max) aerobic bacterial counts for nine trailers at Day 0, Day 1, and Day 2 sampling periods*
* Sampling periods are described in Table 1 and sampling locations in Figure 1 † For each trailer, superscripts represent the IDs of other trailers with significantly different median bacterial counts on Day 1 (compared using the Kruskal-Wallis test [nonparametric ANOVA] followed by Dunn’s multiple comparisons test; P < .05) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bacterial counts by sampling location. Bacterial counts among locations on Days 0, 1, and 2 were not significantly different.
Bacterial isolates
Bacteria from fifteen distinct genera were isolated: Acinetobacter lowffi, Aeromonas spp, Alcalignes spp, Bacillus spp, Enterobacter spp, Enterococcus spp, Escherichia coli, Klebsiella pneumoniae, Moraxella spp, Pantoea agglomerans, Proteus vulgaris, Pseudomonas spp, Staphylococcus spp (coagulase negative), Staphylococcus epidermidis, Stenotropomonas maltophila, and Streptococcus spp (α hemolytic). Enterococcus durans, faecium, and faecolis were identified among the Enterococcus spp. Enterobacter cloacae was identified among the Enterobacter spp. Pseudomonas fluorescens and putida were identified among the Pseudomonas spp.
Discussion
The results of this study suggest that the use of up to 2 days of downtime was not effective in significantly reducing the number of aerobic bacteria isolated from trailers beyond that achieved by cleaning, disinfecting, and drying. Differences in trailer types did not appear to account for major differences in trailer bacterial contamination levels. Moreover, aerobic bacterial counts did not differ among locations sampled within each trailer.
Despite numerical differences, downtime after disinfection did not significantly increase the number of trailers meeting the recommended criterion for disinfection.10 The authors note that the recommendation for disinfection, although published, is arbitrary. For example, the bacteria identified at sampling points during this trial were not considered to be pathogens of adult swine. Thus, increased aerobic bacterial counts are of no concern if the bacteria isolated are not swine pathogens. In this study, the authors did not attempt to use specialized media to isolate specific pathogens. However, the authors suggest that production units establish their own standards of disinfection based on specific targeted pathogens. The goal of the program should be to reduce the level of the targeted pathogens to below the infectious dose of each one.
This study was limited because only nine trailers were sampled during one season of the year. Bacterial counts might vary with climate and a larger sample size might have allowed us to detect significant differences among downtime periods. Downtimes tested in this study were limited to 2 days, which is practical for the industry. Use of longer downtimes might have impacted results. Finally, attempts were not made to isolate specific viral or bacterial pathogens of swine; thus, the impact of downtime on specific agents could not be determined.
Implication
- This study provides no evidence to recommend use of downtimes in vehicle decontamination protocols.
Acknowledgements
Pig Improvement Company (PIC) USA provided financial support for this project. The authors thank Johnny Bell, Tommy Graves, Gary Leitschuck, Ole Torgersen, and Sarah Jensen for their hard work and technical assistance during this project. Finally, the authors thank Dr Joann Kinyon of the Iowa State University Veterinary Diagnostic Laboratory for her technical assistance in identification of bacterial isolates.
Dr Bob Thompson was employed by PIC USA during the study.
References
1. Fritzemeier J, Teuffert J, Greiser-Wilke I, Staubach C, Schlüter H, Moennig V. Epidemiology of classical swine fever in Germany in the 1990s. Vet Microbiol. 2000;77:29–41.
2. Hege R, Zimmermann W, Scheidegger R, Stärk KDC. Incidence of reinfections with Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae in pig farms located in respiratory-disease-free regions of Switzerland – Identification and quantification of risk factors. Acta Vet Scand. 2002;43:145–156.
3. Fussing V, Barfod K, Nielsen R, Møller K, Nielsen JP, Wegener HC, Bisgaard M. Evaluation and application of ribotyping for epidemiological studies of Actinobacillus pleuropneumoniae in Denmark. Vet Microbiol. 1998;62:145–162.
4. Gebreyes WA, Davies PR, Turkson P-K, Morrow WEM, Funk J, Altier C. Salmonella enterica serovars from pigs on farms and after slaughter and validity of using bacteriologic data to define herd Salmonella status. J Food Protect. 2004;67:691–697.
5. Fedorka-Cray PJ, Hogg A, Gray JT, Lorenzen K, Velasquez J, Von Behren P. Feed and feed trucks as sources of Salmonella contamination in swine. Swine Health Prod. 1997;5:189–193.
6. Rajkowski KT, Eblen S, Laubauch C. Efficacy of washing and sanitizing trailers used for swine transport in reduction of Salmonella and Escherichia coli. J Food Protect. 1998;61:31–35.
7. Dee S, Deen J, Rossow K, Weise C, Eliason R, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus through a coordinated sequence of events during warm weather. Can J Vet Res. 2003;67:12–19.
8. Dee S, Deen J, Rossow K, Weise C, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus through a coordinated sequence of events during cold weather. Can J Vet Res. 2002;66:232–239.
9. Dee S, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128–133.
10. Böhm R. Disinfection and hygiene in the veterinary field and disinfection of animal houses and transport vehicles. Int Biodeterioration Biodegradation. 1998;41:217–224.
11. Ford WB. Disinfection procedures for personnel and vehicles entering and leaving contaminated premises. Rev sci tech Off Int Epi. 1995;14:393–401.
12. Poumian AM. Disinfection of trucks and trailers. Rev sci tech Off Int Epi. 1995;14:171–176.
13. Dee S, Deen J, Burns D, Douthit G, Pijoan C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:208–214.
14. Dee S, Torremorell M, Thompson B, Deen J, Pijoan C. An evaluation of thermo-assisted drying and decontamination for the elimination of porcine reproductive and respiratory syndrome virus from contaminated livestock transport vehicles. Can J Vet Res. 2005;69:58–63.
15. Dee S, Deen J, Burns D, Douthit G, Pijoan C. An evaluation of disinfectants for the sanitation of porcine reproductive and respiratory syndrome virus-contaminated transport vehicles at cold temperatures. Can J Vet Res. 2005;69:58–63.
16. Dee S, Deen J. Evaluation of an industry-based sanitation protocol for transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2006;14:126–132.
17. Kahrs RF. General disinfection guidelines. Rev sci tech Off Int Epi. 1995;14:105–122.