Dee SA, Torremorell M, Thompson R, et al. Evaluation of the thermo-assisted drying and decontamination system for sanitation of a full-size transport vehicle contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2007;15(1):12–18
| Original research | Peer reviewed |
Cite as: Dee SA, Torremorell M, Thompson R, et al. Evaluation of the thermo-assisted drying and decontamination system for sanitation of a full-size transport vehicle contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2007;15(1):12–18.
Also available as a PDF.
SummaryObjectives: To determine whether full-size livestock trailers serve as a source of porcine reproductive and respiratory syndrome virus (PRRSV) infection for naive swine, to test the thermo-assisted drying and decontamination (TADD) system in full-size trailers, and to test a method to quantify moisture in trailers post TADD. Methods: Five donor pigs infected with PRRSV (Day 0) were confined within a full-size trailer for a 4-hour contamination period on Days 3 to 7 (five replicates). After each contamination period, a group of five PRRSV-naive recipients (25 pigs total) replaced the donor pigs in the pen for 4 hours. The pen was not washed or sanitized between donor and recipient groups. Recipients were tested for PRRSV by reverse transcriptase-polymerase chain reaction (RT-PCR) post exposure. To test the efficacy of TADD, the trailer was washed, 15 pre-selected interior sites were contaminated with PRRSV, and the TADD system was applied for 2 hours. Swabs collected from the sites at 0, 60, and 120 minutes post treatment were tested by RT-PCR and swine bioassay. A drag-swab technique was developed to measure residual moisture post TADD. Results: Infection of recipient pigs was observed in three replicates. At 0 and 60 but not 120 minutes post TADD, trailer swabs were RT-PCR-positive. Moisture in the drag swabs differed (P < .001) at 0, 60, and 120 minutes post TADD. Implications: Under the conditions of this study, PRRSV-contaminated transport vehicles are a source of infection for naive pigs. The TADD system is efficacious for decontaminating and drying PRRSV-positive trailers. | ResumenObjetivos: Determinar si los traileres de transporte de animales sirven como una fuente de infección del virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) en cerdos libres, probar el sistema de secado y descontaminación con ayuda térmica (TADD sus siglas en inglés) en traileres, y probar un método para cuantificar la humedad en traileres después del TADD. Métodos: Se confinaron cinco cerdos donantes infectados con el PRRSV (Día 0) dentro de un trailer por un periodo de contaminación de 4 horas en los Días 3 a 7 (cinco réplicas). Después de cada periodo de contaminación, un grupo de cinco receptores libres de PRRSV (25 cerdos en total) sustituyeron por 4 horas a los cerdos donantes en el corral. El corral no se lavó ni desinfectó entre los grupos de donantes y receptores. Después de ser expuestos se probaron a los receptores en busca del PRRSV mediante la reacción en cadena de la polimerasa por transcriptasa reversa (RT-PCR por sus siglas en inglés). Para probar la eficacia del TADD, se lavó el trailer, se contaminaron 15 sitios interiores preseleccionados con el PRRSV, y se aplicó el sistema TADD por 2 horas. Se analizaron los hisopos recolectados de los sitios a 0, 60, y 120 minutos post tratamiento con la RT-PCR y bioensayo porcino. Se desarrolló una técnica de arrastre de hisopo para medir la humedad residual post TADD. Resultados: Se observó la infección de los cerdos receptores en tres réplicas. A 0 y 60 pero no a los 120minutos post TADD, los hisopos del trailer fueron RT-PCR-positivos. La humedad en los hisopos de arrastre difirió (P < .001) a 0, 60, y 120 minutos post TADD. Implicaciones: Bajo las condiciones de este estudio, los vehículos de transporte contaminados con el PRRSV son una fuente de infección para cerdos libres. El sistema TADD es eficaz para descontaminar y secar traileres PRRSV positivos.
| ResuméObjectifs: Déterminer si les remorques commerciales utilisées pour le transport des animaux peuvent être la source d’infection par le virus du syndrome reproducteur et respiratoire porcin (PRRSV) pour des porcs naïfs, tester un système d’assèchement thermo-assisté et de décontamination (TADD) pour des remorques commerciales, et tester une méthode pour mesurer l’humidité dans les remorques post-TADD. Méthodes: Cinq porcs disséminateurs ont été infectés avec le PRRSV (Jour 0) et confinés à l’intérieur d’une remorque commerciale durant une période de contamination de 4 heures aux Jours 3 à 7 (cinq réplications). Après chaque période de contamination, un groupe de cinq porcs receveurs, naïfs pour le PRRSV (25 porcs au total), remplaçait les porcs donneurs dans l’enclos pendant quatre heures. Le parc n’était pas lavé ni désinfecté entre la présence des groupes donneurs et receveurs. Les animaux receveurs ont été testés post-exposition pour la présence de PRRSV par réaction d’amplification en chaîne par la polymérase avec la transcriptase inverse (RT-PCR). Afin de vérifier l’efficacité du système TADD, la remorque a été lavée, 15 sites intérieurs pré-sélectionnés ont été contaminés avec du PRRSV, et le système TADD appliqué pendant 2 heures. Des écouvillons prélevés aux sites contaminés 0, 60, et 120 minutes post-traitement ont été testés par RT-PCR et bio-essais chez des porcs. Une technique utilisant des chiffonettes de prélèvement a été développée afin de mesurer l’humidité résiduelle post-TADD. Résultats: L’infection des porcs receveurs a été notée lors des trois essais. Les écouvillonnages de la remorque aux temps 0 et 60 minutes post-TADD, mais pas120 minutes, se sont avérés positifs par RT-PCR. L’humidité mesurée à partir des chiffonettes de prélèvement était significativement différente (P < .001) à 0, 60, et 120 minutes post-TADD. Implications: Dans les conditions expérimentales de la présente étude, les véhicules de transport contaminés par le PRRSV sont une source d’infection pour les porcs naïfs. Le système TADD est efficace pour décontaminer et sécher les remorques positives pour la présence de PRRSV. |
Keywords: swine, porcine
reproductive and respiratory syndrome virus, PRRSV, thermo-assisted drying
and decontamination system, biosecurity
Search the AASV web site
for pages with similar keywords.
Received: December
23, 2005
Accepted: April
10, 2006
Porcine reproductive and respiratory syndrome virus (PRRSV) is a single-stranded enveloped RNA virus classified in the order Nidovirales, family Arteriviridae and genus Arterivirus.1 Over the years, porcine reproductive and respiratory syndrome (PRRS) has proven to be a very difficult disease to control consistently across farms. While PRRSV can be eliminated from infected herds through a number of methods, re-infection with different variants of PRRSV is a frequent event, and the route of viral entry to the farm is often difficult to ascertain. During the last 3 years, extensive efforts to identify routes of PRRSV transmission between swine farms have been attempted. Reported routes of PRRSV transmission include infected pigs, semen, contaminated fomites, insects, avian species, and aerosols.2-8
Another potential route of PRRSV transmission between farms may be the livestock transport vehicle. The process of animal transportation has long been considered an important risk factor for pathogen entry into swine farms, through contact of naive pigs with the interiors of contaminated transport vehicles.9 With the advent of multi-site production, the economics of finishing pigs in specific regions of North America, and the location of the North American packing industry, transport vehicles carry pigs from farm to farm, across state and international borders, or to the slaughterhouse with increasing frequency. Recently, a scientific assessment of the role of the transport vehicle in the spread of PRRSV was conducted using scale models of weaned-pig trailers.10 Under the conditions of that study, it was demonstrated that PRRSV-naive swine could become infected with PRRSV through contact with the contaminated interior of the transport models. It was also determined that the concentration of PRRSV required to infect naive sentinel pigs was ≥ 1 × 103 median tissue culture infectious doses (TCID50), and that allowing the trailer to completely dry after washing effectively prevented infection in 10 of 10 replicates. An adaptation of the drying concept is the thermo-assisted drying and decontamination (TADD) system developed by the Pig Improvement Company (PIC; Franklin, Kentucky). The TADD system is based on the hypothesis that enhanced drying of a PRRSV-contaminated surface results in elimination of residual virus. The TADD system was recently tested using scale models of weaned-pig trailers and was highly effective at eliminating PRRSV from the interior of 10 of 10 replicates.11
While the results were interesting, an acknowledged limitation of the previous studies was the use of trailer models instead of full-size vehicles to demonstrate transmission of PRRSV to naive pigs and to validate the use of the TADD system in a commercial livestock vehicle. Furthermore, the TADD system relies on the absence of moisture in the trailer interior to inactivate PRRSV, yet apart from visual inspection, there is no objective way to assess the effectiveness of the drying process. Therefore, the objectives of this study were to demonstrate the ability of a full-size transport vehicle to serve as a source of PRRSV infection to naive swine, to test the TADD system in a full-size livestock trailer, and to develop and test a method to quantify residual moisture in a trailer following application of the TADD system.
Materials and methods
Description of trailer. For all experiments, an aluminum livestock trailer was employed (EBY Livestock Trailers, Blue Ball, Pennsylvania) that was 18 m in length and 2.7 m in both height and width and consisted of an upper and lower level (double-decked). A loading ramp (11 steps) was fastened inside the trailer to facilitate animal movement between the two levels. Each level contained three hinged gates that could be used to form equal-sized pens, each 1.2 m × 1.2 m (1.45 m2). Pigs were allocated approximately 3 m2 of pen space. During the study, the trailer was housed out of doors at the University of Minnesota Swine Disease Eradication Center research farm in west central Minnesota during the month of August 2005.
Experiment One: Transmission of PRRSV
Animals and housing. A total of fifty 25-kg pigs were purchased from a farm designated PRRSV-free on the basis of 10 years of production and diagnostic data. All animals were cared for under the approved guidelines of the University of Minnesota Institutional Animal Care and Use Committee. Pigs were housed in a commercial nursery facility having power ventilation and pens with wire flooring, nipple water drinkers, and fenceline feeders.
Experimental design. Upon arrival, pigs were randomly assigned to groups. Blood samples were collected and tested by TaqMan polymerase chain reaction (Perkin Elmer Applied Biosystems, Foster City, California)12 and Idexx 2X-R ELISA (Idexx Laboratories, Westbrook, Maine) to ensure a negative PRRSV status. Upon receipt of negative results, five “donor” pigs were infected with PRRSV MN-30100 (Day 0) at a total concentration of 1 × 102.4 TCID50 via the intranasal route.13-15 To ensure that donor pigs were shedding PRRSV, nasal swabs collected from each animal on Days 3 to 7 were tested by reverse transcriptase-polymerase chain reaction (RT-PCR). Five replicates of the transmission trial were conducted, with five groups of five naive pigs acting as recipients (25 recipients total). Ten pigs served as controls. Five control pigs (control donors) were sham-inoculated with sterile cell-culture fluid (minimum essential medium [MEM]; Difco, Detroit, Michigan), while the remaining five pigs served as control recipients.
On each of Days 3 to 7, the PRRSV-infected donor pigs were housed for a 4-hour “contamination” period in a pen on the lower level of the trailer. Immediately afterward, the donors were removed, and a group of recipient pigs entered the trailer and occupied the contaminated pen for a 4-hour exposure period. Pens were designed to allow donor and recipient pigs to enter and exit the trailer by different doors. No attempt was made to remove feces or sanitize the pen between donors and recipients. After the exposure period, each recipient group was placed in a single pen in an isolated room, with no contact with other recipient groups. Donor and recipient pigs were housed in separate buildings 90 m apart.
Diagnostic testing for PRRSV. Nasal samples for RT-PCR were collected by inserting a sterile Dacron cotton swab (Fisher Scientific, Hanover Park, Illinois) 2.54 cm into the nares of each pig, and placing the swab in a plastic tube containing MEM. The quantity of PRRSV RNA in nasal swab samples was determined using a quantitative RT-PCR kit at the University of Minnesota Veterinary Diagnostic Laboratory and a procedure modified from a previously published protocol.12 Data were expressed as TCID50 per mL. A standard curve was developed for the quantitative PCR procedure by preparing 10-fold dilutions of PRRSV MN-30100, ranging from 1 × 106 to 1 × 104.6 TCID50 per mL. Samples were assayed in triplicate, and mean RNA concentrations were calculated from these values.
For the purpose of monitoring the PRRSV status of facilities, swabs were collected from the floor and pen dividers between groups and tested by qualitative PCR. Sterile cotton Dacron swabs were dragged across the respective surfaces using a zig-zag swabbing pattern, then placed into plastic tubes containing MEM.
Experiment Two: Validation of the TADD system in a
full-size
livestock trailer
Trailer preparation. The trailer described in Experiment One was parked on a hill, allowing for a 3% slope to facilitate drainage post washing. To maximize retention of heat throughout this experiment, the sidewall openings of the trailer were covered using “winter panels,” which were pieces of corrugated cardboard (0.3 m × 2.7 m) specifically used for cold-weather animal transport to minimize heat loss from the trailer interior. The first opening on each side of the front of the trailer was left uncovered to allow for air to exhaust.
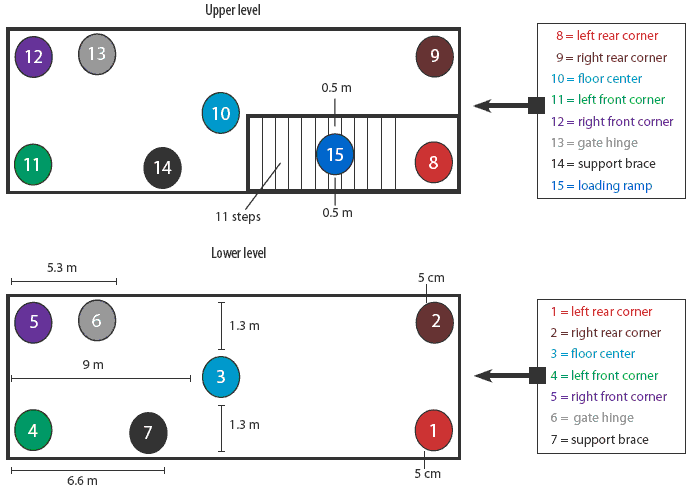
Trailer contamination protocol. The trailer was first washed for 10 minutes using a commercial power washer capable of delivering water at 21°C and 10,500 kPa (American Made Cleaners, Beresford, South Dakota) until all surfaces were visibly wet. Then 5 mL of IngelVac PRRS MLV vaccine (5 × 105 TCID50 total concentration; Boehringer Ingelheim Vetmedica, St Joseph, Missouri) was applied to each of 15 sites throughout the trailer (Figure 1). Sites included the left rear, right rear, left front, and right front corners on both the upper and lower levels; the middle of the floor on the lower and upper levels; the hinge on the gate closest to the front of the trailer; one of the ceiling support braces on each level; and the loading ramp. At each site, the vaccine was spread over an area approximately 5 cm × 5 cm. For inoculation of the corners, each 5-mL aliquot of vaccine was placed on the floor 5 cm from the contributing sides of each corner. For inoculation of the middle of the floor, a 5-mL aliquot of vaccine was placed on a point exactly 1.3 m from either side of the trailer and 9 m from the front and rear walls. The second gate hinge on each level, located 5.3 m from the front end of the trailer and approximately 0.4 m off the floor, was inoculated by dripping the 5 mL of vaccine onto the interior of the hinge. The ceiling support brace on each level was inoculated by expelling the 5 mL of vaccine onto the top of the brace, approximately 6.6 m from the front end of the trailer and 1 m off the floor. Finally, 5 mL of vaccine was placed on the middle of the loading ramp (step number six), approximately 0.5 m from either edge of the ramp. Immediately after inoculation of each site, the inoculated area was swabbed and swabs were stored and tested by qualitative PCR.
| Figure 1: Diagram showing sites where PRRSV MLV
vaccine was applied in both levels of a full-size double-decked trailer
used in an evaluation of the thermo-assisted drying and decontamination
system. A standard amount (5 mL) and concentration (5 × 105 median
tissue culture infectious doses) was applied to each site.
|
Application of the TADD system. Ten replicates were conducted, each consisting of a 2-hour treatment period. High velocity air (12.4 m per second) at 88°C to 92°C was applied to the trailer interior using a Chinook heater (MAC Inc, Glenburn, North Dakota) capable of generating 1.2 million BTU of heat each hour. To deliver the air from the heater into the trailer interior, two flexible pipes (0.3 m diameter, 6.6 m long) were placed into the trailer via the rear doors. On the lower level, one pipe was fully extended and placed flat on the floor. For treatment of the upper level, the loading ramp was extended from the upper to the lower level at a 45-degree angle. The second pipe was then fully extended up the ramp and down the length of the floor. After 60 minutes of treatment, the 15 inoculated sites were swabbed. For the second hour of treatment, the lower level pipe was retracted to a length of 1 m, while the upper level pipe remained on the loading ramp but was retracted to a length of 4 m. Immediately before the end of the 2-hour drying period in each replicate, floor temperatures on both the upper and lower levels of the trailer were determined using a hand-held infra red thermometer. (Raytek MT Mini-temp; Raytek, Santa Cruz, California). The instrument was held perpendicular to the floor at an elevation of 15 cm from the floor. Readings were taken at designated points approximately 1 m, 9 m, and 17 m from the front of the trailer on both the upper and lower levels. Finally, at the end of the 2-hour treatment period, the 15 sites were swabbed again and tested for PRRSV by quantitative RT-PCR as described.
Diagnostic testing. All swabs were tested for PRRSV RNA by TaqMan PCR.12 To determine if a PCR-positive sample contained infectious PRRSV, a modified swine bioassay protocol was conducted.16 For this procedure, 1-mL aliquots of supernatant from each of 10 PCR-positive swab samples were pooled, and this volume was injected intramuscularly into a PRRSV-naive pig. An additional set of 10 “bioassay” pigs were housed in separate facilities to prevent nose-to-nose contact. Blood was collected from each bioassay pig 7 and 14 days post inoculation, and serum was tested by TaqMan qualitative PCR for PRRSV and by Idexx 2X-R ELISA for antibodies to PRRSV.
Controls. On each experimental day, prior to application of the TADD system, the trailer was also used as a positive and negative control. For the negative controls, all 15 sites were sham-inoculated with sterile saline and samples were collected and tested as described. For positive controls, the 15 sites were inoculated with vaccine and sampled as described at 0, 60, and 120 minutes without using the TADD treatment.
Experiment Three: Development of a protocol to quantify residual moisture in a full-size trailer post TADD
To quantify residual moisture in the interior of treated trailers, a drag-swab technique was developed. A long sheet of cotton fabric (WypAll X70; Kimberely-Clark, Roswell, Georgia; 23 cm × 42 cm) was dragged around the edges of the lower and upper level floors of the trailer in a circular clockwise pattern, using a progressively smaller diameter until the entire floor surface had been sampled. To facilitate the swabbing procedure, the drag swab was stapled to a wooden paint stir stick (0.3 m long). A 2-mm hole was drilled in the middle of the stick, through which a 1-m string was tied to allow the swab to be dragged over the floor surface (Figure 2). Drag swabs were collected at 0, 60, and 120 minutes post treatment in all treatment and positive-control replicates. A new drag swab was used for each replicate. To determine the amount of moisture at each sampling period, the drag swab was weighed using a platform balance before being stapled to the stick and after swabbing. After swabbing, the drag swab was removed from the stick, placed in a plastic container, and weighed on a kitchen scale (Figure 3). The balance was calibrated each study day and re-calibrated between replicates. For the purpose of a negative control, swabs were collected from dry trailers at the beginning of each study day.
| Figure 2: Drag-swab method used to assess residual
moisture in a pig transport trailer treated using the thermo-assisted drying
and decontamination system. The drag swab was a sheet of cotton fabric
(23 cm × 42 cm) stapled to a wooden paint stir stick (0.3 m long).
A 1-m string tied through a 2-mm hole drilled in the middle of the stick
allowed the swab to be dragged over the floor surface of the trailer.
|
| Figure 3: Method for calculating grams of residual
moisture collected in a drag swab that had been weighed dry, dragged over
the floor surface of a pig transport trailer (Figure 2), then placed in
the bucket of a kitchen scale and weighed again.
|
Data analysis
The number of PCR-positive swabs and the quantities of moisture collected from treated trailers and positive-control trailers at 0, 60, and 120 minutes post TADD were compared in a Kruskal-Wallis one-way nonparametric ANOVA, with α set at 0.05.
Results
Experiment One: Transmission of PRRSV to naive pigs
PRRS virus RNA was detected in nasal swab samples from all donor pigs on Days 3 to 7. The concentration of RNA detected across all donors averaged 1 TCID50 per mL on Day 3 (range = 0 to 3.4 TCID50 per mL), 1.5 TCID50 per mL on Day 4 (range = 0 to 3.6 TCID50 per mL), 69 TCID50 per mL on Day 5 (range = 26 to 201 TCID50 per mL), 48 TCID50 per mL on Day 6 (range = 2 to 58 TCID50 per mL), and 29 TCID50 per mL on Day 7 (range = 6 to 92 TCID50 per mL). Evidence of transmission of PRRSV to naive recipient pigs was observed in three of five groups, occurring on Days 5, 6, and 7. Sham-inoculated donors and recipient control pigs remained PCR-negative, and all swabs collected from the sanitized pen between the five replicates were PCR-negative. Nucleic acid sequencing of the ORF 5 region of three PRRSV isolates recovered from donors (n = one isolate), recipients (n = one isolate), and the original inoculum (n = one isolate) indicated 99.5% homology across samples.
Experiment Two: Validation of the TADD system in a full-size livestock trailer
This experiment was conducted over a 5-day period, with two replicates conducted each day. According to weather data from a nearby airport, the environmental temperature of the farm area during this time averaged 21.4°C (range = 17°C to 25°C) and relative humidity averaged 80% (rangen = 67% to 95%). After completion of the 2-hour drying process, the mean floor temperatures across the 15 replicates on the upper level, 1 m, 9 m, and 17 m from the front of the trailer, were 45°C (range = 43° C to 47°C), 50°C (range = 48°C to 53°C), and 43°C (range = 39°C to 46°C), respectively. On the lower level, the mean floor temperatures across the 15 replicates 1 m, 9 m, and 17 m from the front of the trailer were 40°C (range = 37°C to 42°C), 37°C (range = 36°C to 39°C), and 34°C (range = 31°C to 37°C), respectively. A total of 150 swabs were collected across all 10 replicates (15 swabs per replicate). At 0 minutes, 150 of 150 of the swabs (100%) were PCR-positive. Due to the large number of positive samples, one positive sample from each replicate (n = 15 total samples) was selected at random and these 15 samples were pooled. This pool was bioassay-positive. At 60 minutes, 35 of 150 swabs (23.3%) were PCR-positive; however, all samples (pooled 10:1 as described) were swine bioassay-negative. Finally, at 120 minutes, all swabs (150 of 150) were PCR-negative. The difference between the numbers of PCR-positive swabs collected at 0 and 60 minutes was significant (P < .001), as was the difference between the number of PCR-positive swabs collected at 60 and 120 minutes (P < .001). The percentage of PCR-positive samples collected from positive controls across all replicates (n = 5) was 80% (60 of 75 samples) at 60 minutes and 53% (40 of 75 samples) at 120 minutes. The difference between the percentages of PCR-positive swabs in the treated trailer and the positive-control trailer at 60 and 120 minutes was significant (P < .001). Pooled samples from the positive-control trailer were swine bioassay-positive at 60 and 120 minutes. All samples from all negative-control replicates (n = 5) were PCR-negative.
Experiment Three: Development of a protocol to quantify residual moisture in a full-size trailer post-TADD treatment
The amount of moisture in the drag-swab from the treated trailer
averaged 212 g at 0 minutes (range = 180 to 235 g, SE = 6.5 g),
46 g at 60 minutes (range = 15 to 78 g, SE = 6.3 g), and 0 g at 120
minutes. The difference in quantities of moisture at each time
within treated trailers and between treated trailers when compared
to the positive controls was significant (P < .001). In
contrast, the amount of moisture in drag-swabs from
positive-control replicates averaged 203 g at 0 minutes (range =
192 to 221 g, SE= 5.2 g), 126 g at 60 minutes (range = 96 to 147,
SE = 9.7 g) and 83 g (range = 65 to 102, SE= 6.8 g) at 120 minutes.
The quantity of moisture detected in drag swabs from negative
controls was 0 g across all replicates.
Discussion
The objectives of this study were to re-evaluate the transmission of PRRSV to naive pigs following contact with the interior of contaminated transport vehicles, to assess the efficacy of the TADD system for reducing the quantity of PRRSV in a trailer, and to evaluate a means for quantifying residual moisture post TADD. The primary difference between this study and previous studies evaluating the role of transport in PRRSV transmission and transport bio-security10,11 was the use of a full-size transport vehicle. The results of Experiment One support previous data indicating that contaminated transport vehicles can indeed serve as a source of infection to naive pigs.10 While this is not new information, it does demonstrate that transmission is possible in full-size trailers, supporting the previously published observations in scale-model weaned-pig trailers.10 Furthermore, the use of a full-size trailer was much more representative of commercial conditions, and the space allotted to the animals allowed them to move about the pen, in contrast to the scale models where no movement was possible. It was interesting to observe that transmission to recipient pigs following contact with PRRSV-contaminated trailer interiors occurred during the latter portion of the exposure period (days 5 to 7 post infection of donors). An explanation for the lack of transmission earlier in the exposure period may be that donor animals had not been infected long enough to be capable of shedding sufficient quantities of virus into the environment to infect the recipients. However, this was a numerical observation only, and was not evaluated statistically.
The results of Experiment Two also support previously published data in scale-model trailers, indicating that the TADD system is a highly effective means for removing moisture from a full-size trailer as well as significantly reducing the level of PRRSV within contaminated trailer interiors.11 In today’s commercial swine industry, rapid (≤ 2 hours) efficacious sanitation protocols for transport vehicles are critical to reduce the risk of PRRSV transmission by transport. The fact that samples were bioassay-negative at 60 minutes post treatment suggests that treatment periods of < 120 minutes may be effective at decontaminating PRRSV-positive trailers. However, this may not be consistently observed under different weather conditions, eg, in cold wet climates. Therefore, future research should validate the efficacy of the TADD system under varying environmental conditions and against other swine pathogens, such as transmissible gastroenteritis virus or Escherichia coli.
Concurrent with Experiment Two, this study also attempted to develop a means to quantify the amount of residual moisture in full-size trailers after washing and application of the TADD system (Experiment Three). This experiment was conducted to demonstrate the ability of the TADD system to consistently dry trailers. Since it has been documented11 that drying inactivates PRRSV, the ability to employ a system of validating the level of residual moisture in a treated trailer is critical for quality control and assessment of personnel compliance. These results indicate that the drag-swab method can detect significant differences in the level of moisture in washed trailers over time and that the amount of moisture (0 g) detected at 120 minutes post treatment was equal to that detected in negative controls. Application of this monitoring protocol may be a means to determine if a treated trailer is truly dry and thereby safe to use.
This study had a number of strengths and limitations. Strengths included use of an aggressive challenge model, a trailer representative of commercial swine conditions, a large number of replicates, and multiple tests to assist in the interpretation of diagnostic data. This is the first report of a full-size livestock vehicle being used to test the ability of contaminated transport vehicles to transmit PRRSV to naive pigs and to evaluate PRRSV sanitation protocols. The study also highlights the importance of drying in the sanitation process for transport vehicles, especially when PRRSV is involved. Acknowledged limitations include inability to use large numbers of animals in Experiment One or extensive replication of Experiments 2 and 3, and a lack of debris (eg, feces, wood shavings) in the interior of the trailer. Transport vehicles of this size typically haul 200 to 225 market-weight animals, and on unloading, the interior is always soiled with urine and feces from the animals and bedding used during transport. Under field conditions, this material may hinder the sanitation process; however, we decided to start with a washed trailer that was free of debris. The contamination protocol, while aggressive, was highly artificial and used concentrations of virus that are not representative of field conditions. However, it has been previously determined11 that sentinel pigs can be infected with PRRSV in model trailers contaminated with concentrations of ≥ 1 × 103 TCID50, supporting the use of this concentration. In this study, modified-live vaccine virus was used instead of field virus. While this could be considered a limitation, it is unlikely that the biochemical properties of the vaccine virus would differ from those of the field virus to the degree that the results would be influenced.
Under the conditions of this study, the TADD system successfully dried and decontaminated full-size PRRSV-positive transport vehicles. Therefore, on the basis of the information generated through these experiments, it is hoped that swine producers and practitioners will continue to understand and appreciate the merit of drying for sanitation of livestock transport vehicles, to enhance the level of biosecurity and protection of their farms.
Implications
- Contaminated transport trailer interiors can serve as a source of PRRSV infection for naive pigs.
- The TADD system is an effective means for reducing the quantity of PRRSV in contaminated trailers.
- The use of drag swabs to assess the presence of residual moisture post TADD may be a means to monitor the quality control of a transport biosecurity program.
Acknowledgements
Resources for this study were made available by PIC and the National Pork Board PRRS Initiative. Dr Thompson and Dr Torremorell are employed by PIC USA.
References
1. Cavanagh N. Nidovirales. A new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633.
2. Dee SA, Joo HS, Pijoan C. Controlling the spread of PRRS virus in the breeding herd through management of the gilt pool. Swine Health Prod. 1995;3:64–69.
3. Christopher-Hennings J, Nelson EA, Hines RJ, Nelson JK, Swenson SL, Zimmerman JJ, Chase CCL, Yaeger MJ, Benfield DA. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456–464.
4. Otake S, Dee SA, Rossow KD, Joo HS, Deen J, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus by fomites (boots and coveralls). Swine Health Prod. 2002;10:59–65.
5. Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus by mosquitoes; Aedes vexans (Meigen). Can J Vet Res. 2002;66:191–195.
6. Otake S, Dee SA, Rossow KD, Moon RD, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica). Vet Rec. 2003;152:73–76.
7. Zimmerman JJ, Yoon KJ, Pirtle EC, Wills RW, Sanderson TJ, McGinely MJ. Studies of porcine reproductive and respiratory syndrome (PRRS) virus in avian species. Vet Microbiol. 1997;55:329–336.
8. Torremorell M, Pijoan C, Janni K, Walker R, Joo HS. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Am J Vet Res. 1997;58:828–832.
9. Poumian AM. Disinfection of trucks and trailers. Rev sci tech Off Int Epiz. 1995;14:171–176.
10. Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128–133.
11. Dee SA, Torremorell M, Thompson B, Deen J, Pijoan C. An evaluation of the Thermo-Assisted Drying and Decontamination (TADD) system for the elimination of porcine reproductive and respiratory syndrome virus from contaminated livestock transport vehicles. Can Vet J Res. 2005;69:58–63.
*12. Molitor TW, Tune KA, Shin J, Collins J, Kapur V. Applications of TaqMan™ PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf. 1997;173–175.
13. Bierk MD, Dee SA, Rossow KD, Collins JE, Guedes MI, Pijoan C, Molitor TW. Diagnostic investigation of chronic porcine reproductive and respiratory syndrome virus infection in a breeding herd of pigs. Vet Rec. 2001;148:687–690.
14. Cho JG, Dee SA, Deen J, Guedes A, Trincada C, Fano E, Jiang Y, Faaberg K, Collins JE, Murtaugh MP, Joo HS. Evaluation of the effects of animal age, concurrent bacterial infection and pathogenicity of porcine reproductive and respiratory syndrome virus in pigs. Am J Vet Res. 2006;67:489–493.
15. Cho JG, Dee SA, Deen J. Evaluation of isolate pathogenicity on the transmission of porcine reproductive and respiratory syndrome virus in aerosols. Can J Vet Res. In press.
16. Swenson SL, Hill HT, Zimmerman JJ, Evans LE, Landgraf JG, Wills RW, Sanderson TP, McGinley MJ, Brevik AK, Ciszewski DF, Frey ML. Excretion of porcine reproductive and respiratory syndrome virus in semen after experimentally induced infection in boars. JAVMA. 1994;204:1943–1948.
* Non-refereed reference.