Original research |
Peer reviewed |
Sampling of adult boars during early infection with porcine reproductive and respiratory syndrome virus for testing by polymerase chain reaction using a new blood collection technique (blood-swab method)
Muestreo de machos adultos durante la infección temprana con el virus del síndrome reproductivo y respiratorio porcino con la reacción en cadena de la polimerasa utilizando una nueva técnica de recolección de sangre (método de hisopo de sangre)
Prise d‘échantillon chez des verrats adultes tôt durant l‘infection par le virus du syndrome reproducteur et respiratoire porcin pour une épreuve d‘amplification en chaîne par la polymérase utilisant une nouvelle technique de prélèvement de sang (méthode de l‘écouvillon sanguin)
Darwin L. Reicks, DVM; Claudia Muñoz-Zanzi, DVM, MPVM, PhD; Kurt Rossow, DVM, PhD
DLR: Swine Veterinary Center, St Peter, Minnesota. CMZ: Department of Veterinary Population Medicine, University of Minnesota, St Paul, Minnesota. KR: Veterinary Diagnostic Laboratory, University of Minnesota College of Veterinary Medicine, St Paul, Minnesota. Corresponding author: Dr Darwin L. Reicks, 1608 S Minnesota Ave, Box 269, St Peter, MN 56082; Tel: 507-934-3970; Fax: 507-934-3968; E-mail: dreicks@swinevetcenter.com
Cite as: Reicks DL, Muñoz-Zanzi C, Rossow K. Sampling of adult boars during early infection with porcine reproductive and respiratory syndrome virus for testing by polymerase chain reaction using a new blood collection technique (blood-swab method). J Swine Health Prod. 2006;14(5):258-264.
Also available as a PDF.
SummaryObjectives: To evaluate the effectiveness of a skin puncture technique (blood-swab method) to collect blood for detection of porcine reproductive and respiratory syndrome virus (PRRSV) by polymerase chain reaction (PCR) in experimentally infected boars, and to examine the effect of sampling method (blood swab, serum, semen, or mouth swab) and rectal temperature on the ability to detect PRRSV in boars during the first 6 days after experimental infection. Materials and methods: Twenty-one mature boars were inoculated with PRRSV variant MNB04. Serum samples, blood swabs, mouth swabs, and semen samples were collected from two groups of 10 boars on a rotating basis, one group every 24 hours for 6 days. Rectal temperatures were recorded for all boars every 24 hours. Using the blood-swab method, a vein (usually the auricular vein) was punctured with a needle and blood was collected with a polyester swab. The swab was then put into a tube containing 1 mL of saline solution, which was tested for PRRSV virus by PCR. Results: Sixty of 61 samples were PCR-positive using the blood-swab method compared with 61 of 61 PCR-positive using traditional serum collection methods. Testing of both serum and blood swabs detected PRRS-positive boars earlier and with higher frequency than testing semen samples (27 of 60 positive) or mouth-swab samples (19 of 61 positive). Implications: The blood-swab method is a reliable alternative to traditional venipuncture during the first 6 days after infection with PRRSV, and PCR-testing blood swabs is more sensitive than testing semen or mouth swabs. | ResumenObjetivos: Evaluar la eficacia de una técnica de punción de piel (método de hisopo de sangre) para recolectar sangre para la detección del virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) con la reacción en cadena de la polimerasa (PCR por sus siglas en inglés) en machos infectados experimentalmente, y examinar el efecto de métodos de muestreo (hisopo de sangre, suero, semen, o hisopo de boca) y temperatura rectal en la habilidad para detectar el PRRSV en machos durante los primeros 6 días después de la infección experimental. Materiales y métodos: Se inocularon veintiún machos maduros con el PRRSV variante MNB04. Se recolectaron muestras de suero, hisopos de sangre, hisopos de boca, y muestras de semen de dos grupos de 10 machos en base rotacional, un grupo cada 24 horas por 6 días. Se registraron las temperaturas rectales de todos los machos cada 24 horas. Utilizando el método de hisopo de sangre, se hizo punción de una vena (generalmente la vena auricular) con una aguja y se recolectó la sangre con un hisopo de poliéster. El hisopo se puso entonces en un tubo que contenía 1 mL de solución salina, la cual se probó para ver si contenía el virus de PRRSV por PCR. Resultados: Sesenta de 61 muestras fueron PCR positivas utilizando el método de hisopo de sangre comparado con 61 de 61 PCR positivas utilizando métodos tradicionales de recolección de suero. La prueba tanto de suero como de hisopos de sangre detectó machos antes y con más alta frecuencia que la prueba de muestras de semen (27 de 60 positivas) o muestras de hisopos de boca (19 de 61 positivas). Implicaciones: El método de hisopo de sangre es una alternativa confiable a la punción de vena tradicional durante los 6 primeros días después de la infección con el PRRSV, y la prueba de PCR de hisopos de sangre es más sensible que probar el semen o hisopos de boca. | ResuméObjectifs: Êvaluer l’efficacité d‘une technique de ponction cutanée (méthode de l‘écouvillon sanguin) afin de prélever du sang pour la détection du virus du syndrome reproducteur et respiratoire porcin (PRRSV) par réaction d‘amplification en chaîne par la polymérase (PCR) chez des verrats infectés expérimentalement, et examiner les effets de la méthode d‘échantillonnage (écouvillon sanguin, sérum, semence, ou écouvillon buccal) et de la température rectale sur la capacité à détecter le PRRSV chez des verrats durant les 6 premiers jours suivant une infection expérimentale. Matériels et méthodes: Vingt-et-un verrats matures ont été inoculés avec le variant MNB04 du PRRSV. Des échantillons de sérum, des écouvillons sanguins, des écouvillons buccaux, et des échantillons de semence ont été prélevés en alternance de deux groupes de dix verrats, un groupe chaque 24 heures pendant 6 jours. La température rectale a été enregistrée à chaque 24 heures pour tous les verrats. La méthode de l‘écouvillon sanguin consistait à ponctionner une veine (habituellement la veine auriculaire) à l‘aide d‘une aiguille et à récolter le sang à l‘aide d‘un écouvillon en polyester. L‘écouvillon était par la suite placé dans un tube contenant 1 mL de solution saline, et était par la suite testé pour la présence de PRRSV par PCR. Résultats: Soixante des 61 échantillons étaient positifs par PCR en utilisant la méthode de l‘écouvillon sanguin comparativement à 61 sur 61 lorsque du sérum prélevé de manière traditionnelle était utilisé pour l‘épreuve PCR. L‘analyse du sérum et de l‘écouvillon sanguin a permis de détecter plus rapidement et avec une plus grande fréquence les verrats positifs comparativement à l‘analyse de semence (27 des 60 positifs) ou des écouvillons buccaux (19 des 61 positifs). Implications: La méthode de l‘écouvillon sanguin est une alternative fiable à la ponction veineuse traditionnelle durant les 6 premiers jours de l‘infection par le PRRSV, et l‘analyse par PCR des écouvillons sanguins est plus sensible que l‘analyse de la semence et des écouvillons buccaux. |
Keywords: swine, porcine
reproductive and respiratory syndrome virus, PRRSV, boar, blood, semen
Search the AASV web site
for pages with similar keywords.
Received: June
7, 2005
Accepted: November
7, 2005
Previous studies have shown that porcine reproductive and respiratory syndrome virus (PRRSV) may be shed in semen, and this is a well-accepted fact.1,2 Therefore, it is important that only PRRSV-negative boars enter boar studs, and boar studs should maintain a negative status to minimize the risk of shedding virus to recipient sows. If boars in the studs are infected with PRRSV, it is critical to detect the virus as soon as possible, before virus is distributed to recipient sows through the semen. In the field, boars typically display minimal clinical signs when infected with PRRSV. Thus, polymerase chain reaction (PCR) sampling has become the most common method for detection of PRRSV in boars because it detects viral RNA within days after the animal is infected. Semen PCR has become the standard method of detecting PRRSV in boar studs. The sample is readily available and easy to obtain. In order to obtain serum from boars, restraint is normally required. This may create a safety hazard, and boars may become more difficult to restrain at each additional sampling.
Serum PCR is more sensitive than semen PCR,3 and detects PRRSV by PCR testing within 24 to 48 hours after an animal is infected.3-7 To detect PRRSV-infected boars earlier, serum should be collected rather than semen. Because many boar studs sample boars randomly, collecting blood rather than semen should lessen the chance of the boar stud distributing infectious PRRSV in semen. However, collecting a blood sample by venipuncture each time a boar is collected (once or twice per week) is dangerous and difficult, and semen, the easily available sample, is currently being used to screen boars for PRRSV infection. Because semen PCR testing is the current industry method of testing boars, there might be considerable lag between infection of a boar stud and closure for semen distribution. A sampling technique is needed that is easier than snaring the boar and collecting blood from the jugular or vena cava, so that boars could be easily sampled by the boar stud staff at each semen collection (ie, once or twice weekly per boar). The objective of this study was to develop a new and easy technique to monitor PRRSV-negative boar studs to further minimize the chance of introducing PRRSV into recipient sows through the semen.
Materials and methods
Animals
A total of 21 boars between 10 and 12 months of age were sourced from a PRRS-naive boar stud that is tested monthly by serum PCR for PRRSV and by ELISA for PRRSV antibodies (Idexx HerdChek 2XR ELISA; Idexx Laboratories, Westbrook, Maine), and by semen PCR for PRRSV each collection day. All 21 boars were tested by serum PCR before the beginning of the study to verify their PRRSV-free status. The boars were divided into two groups of 10 boars, with the remaining boar serving as a backup in the event of a death or severe health problem. An additional two working, PRRSV-negative boars from the same source herd (negative controls) were used to obtain negative samples for quality-control purposes and to monitor for cross-contamination that might have occurred during sample handling.
Housing, feeding, and management
After negative PRRS ELISA and PRRSV PCR results were available, the 21 boars were moved to an isolated research facility and allowed to acclimate for 2 days. All boars were housed in individual stalls (1.5 m2) with partially slatted floors, with nose-to-nose contact between boars. All animals were housed in the same room with forced-air ventilation targeted to maintain a temperature of 22°C to 24°C. Water drippers activated when temperatures exceeded 26°C. Animals were fed a corn and soybean-meal diet (16% crude protein) once daily. Semen was collected in one of two identical, adjacent collection pens. Control boars remained in the original facility, which had forced-air ventilation, totally slatted flooring, and the same target temperature range, cooling guidelines, and stall size as the facility housing the principal boars. All animals at both facilities were cared for in accordance with published animal welfare guidelines.8
Experimental design
In order to ensure comparability between groups, 20 boars were assigned randomly to two groups (A and B). All 21 boars were inoculated intranasally with PRRSV on Day 0, with 2.0 mL of inoculum containing 3.2 × 105 median tissue culture infective doses per mL. Semen, serum, and blood-swab samples were collected at various times post inoculation (Table 1). Because of the logistic and animal handling difficulties associated with sampling all boars every day, each group of 10 boars (A or B) was sampled on alternate days during the first 6 days after inoculation. One negative control boar per group was sampled at the same time periods and tested by PCR. To verify infection, all boars were resampled 23 days post inoculation.
Table 1: Sampling schedule for semen, serum, and blood-swab collection for the purpose of detecting porcine reproductive and respiratory syndrome virus (PRRSV) in two groups of boars intranasally inoculated with 2.0 mL of PRRSV variant MNB043 containing 2 × 105 median tissue culture infective doses per mL
* Controls were two working, PRRSV-negative boars from the same source herd. |
|||||||||||||||||||||||||||||||||||||
Inoculum
The inoculum (PRRSV variant MNB04) was prepared by passage of the virus in MARC-145 cells and was suspended in minimal essential medium.
Sample collection and storage
Semen was collected using a dummy and standard procedures. Aliquots of semen were chilled on ice until testing.
Blood was collected for serum samples by traditional venipucture from the anterior vena cava using a 3-inch needle and syringe. Samples were centrifuged on site to separate the serum. Aliquots were stored on ice until testing.
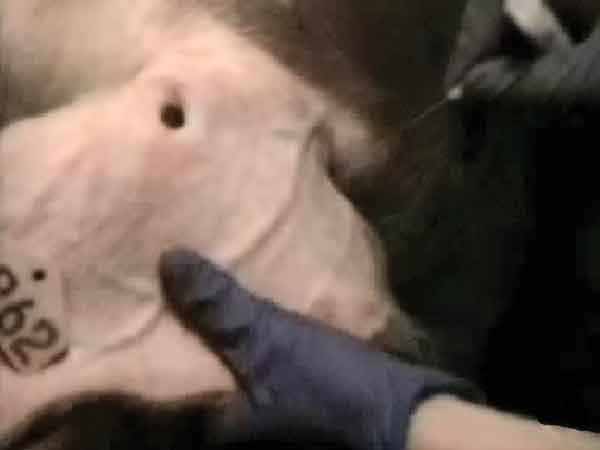
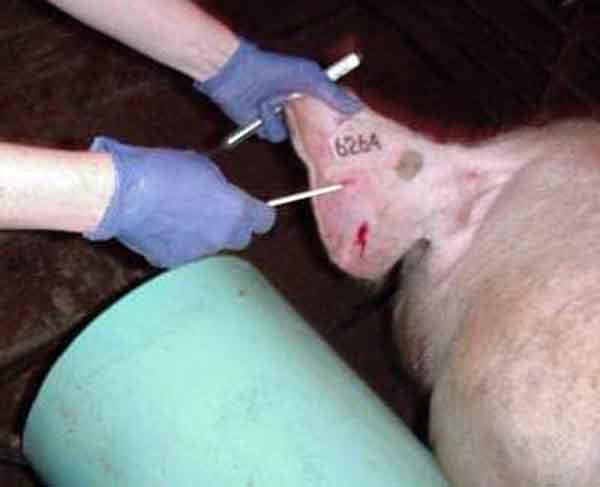
Blood-swab samples were collected using a 20-gauge, 1/2-inch needle, which was used to puncture through the skin and into a visible vein either beside the tail head (perineal vein) or in the ear (auricular vein). For the first blood-swab collection on each boar (24-hour and 48-hour sampling periods), puncture of the perineal vein was attempted. If this was unsuccessful, blood was collected from an auricular vein (Figure 1). For additional sampling times, the auricular vein was punctured after wiping with a dry paper towel. The blood drops were collected from the surface of the skin using a Rayon polyester swab (to the point of saturation). The swab was immersed in a tube containing 1 mL of 0.9% saline solution and stored on ice until testing. All blood was collected during ejaculation while the boar was mounted on the collection dummy (Figure 2).
Figure 1: Locating the auricular vein in a boar during ejaculation, and preparing to puncture with a 20-gauge, 1/2-inch needle.
(Image enhanced for clarity and to reduce noise) |
Figure 2: Blood-swab sampling after puncture of the auricular vein of a boar during ejaculation and while mounted on a collection dummy.
|
Finally, a mouth swab was collected, placed in 1.0 mL of 0.9% saline solution, and stored on ice until testing.
PCR testing
All samples were tested at the University of Minnesota Veterinary Diagnostic Laboratory. For the Taqman PCR, a commercial kit (QIAamp DNA Blood BioRobot 9604 kit; Qiagen, Valencia, California) was used for RNA extractions using 200 uL of the original serum and semen samples, according to the manufacturer‘s protocol. After brief centrifugation to pellet red cells (blood swab) or debris (mouth swab) and removal of the swab, blood-swab and mouth-swab samples were handled in the same manner as serum and semen. An automated viral purification procedure was used for extraction,9 using duplicate samples. One-step reverse transcriptase- (RT-) PCR was then performed on the extracted RNA using a commercial kit (One Step RT-PCR kit; Qiagen), according to the manufacturer‘s protocol. Samples were tested for US PRRSV by Taqman PCR using 5 uL of the extracted RNA to detect a portion of open reading frame 6 of the PRRSV.9 Results were determined to be positive, negative, or suspect. A suspect result was an inconclusive result, (ie, one positive and one negative result on the same sample). Suspect results were considered positive for purposes of statistical analysis.
Quantitative PCR data (measured as viral particles per mL) were obtained using a quantitative PCR for each sample that was initially PCR-positive. Virus concentration was measured by comparison of positive samples to a standard curve generated from plaque assay10 concentrations of PRRSV (Applied Biosystems Software; Applied Biosystems, Foster City, California). Standards and test samples were extracted using an identical procedure, thus the number of infectious viral particles (IVP) per mL was determined on the basis of the starting volume of the original sample.
Serum ELISA
Serum samples were tested for PRRSV antibodies using the Idexx HerdChek PRRS 2XR ELISA (Idexx Laboratories) at the Minnesota Veterinary Diagnostic Laboratory to verify the serological status of the boars before inoculation and 23 days post inoculation. A sample:positive (S:P) ratio < 0.40 was considered negative.
Rectal temperatures
A digital thermometer was calibrated using a calibrated thermometer traceable to National Institute of Science and Technology (NIST) standards. Rectal temperatures were obtained on all boars once daily. Room temperature was also obtained using a high-low thermometer accurate to within 0.6°C when checked using a thermometer certified to NIST standards. Boars with body temperature > 39.4°C were considered to have fever. Room temperature > 23.3°C was designated to have a confounding influence on rectal temperature, determined prior to the start of the trial. Rectal temperatures were not obtained on control boars because they were in a different facility. Clinical signs, such as off-feed and lethargy, were observed but not recorded.
Flinch rate
For welfare considerations, flinch rate was recorded when the blood-swab collection method was used. A flinch was recorded if there was any negative reaction from the boar at the time of the skin puncture with the needle.
Statistical analysis
Descriptive statistics were initially performed to summarize and describe the data on diagnostic testing, pooling, and rectal temperature. Results of PCR testing on the various sample types at different times after inoculation were compared on the basis of the proportion of agreement and 90% confidence interval. The proportion of agreement was calculated for each relevant pair-wise combination of PCR results (serum and blood swab, serum and semen, serum and mouth swab) for each time after inoculation using the following equation: (number of boars positive in both tests + number of boars negative in both tests) ÷ total number of boars tested.11 The 90% confidence interval for each proportion was calculated using exact methods (Statistica 6.0; StatSoft, Tulsa, Oklahoma). In addition, the McNemar‘s test was used to assess the statistical difference between the numbers of positive PCR results for each pair-wise combination at a given sampling time.12 Survival analysis methods, specifically the interval-censored Cox proportional hazards regression model,12 were used to model the time to first detection of PRRSV by PCR in each sample type (S-Plus 6.2; Insightful Corp, Seattle, Washington). Results calculated included median time and 90% confidence interval to the first PCR-positive test (ie, the time when 50% of the pigs were expected to test PCR-positive for the first time). Association between fever and a PCR-positive test was evaluated in the survival model. Fever (yes: ≥ 39.4° C; no: < 39.4°C) was included in the model as a time-dependent variable. The statistical requirement of proportional hazards was evaluated on the basis of the plot of the log (-log (Sˆ )), where Sˆ corresponded to the survival function, and of the plot of the Schoenfeld residuals. Plots of the Cox-Snell residuals were used to assess overall goodness-of-fit for the final model.13 Due to the small sample size, a P value < .10 was considered statistically significant in all analyses.
Results
One boar became ill by 72 hours post inoculation and died as a result of PRRSV infection, according to the pathologist report. He was replaced in the sampling chart with the extra boar that was inoculated at time 0.
Blood-swab samples were successfully obtained at all collection times. Specifically, sampling from the ear vein using the blood-swab method during collection was successful on each attempt (52 of 52). Sampling from the perineal vein was less successful (for nine of 20 attempted, there was enough blood to saturate the swab).
PCR results for serum, blood-swab, mouth-swab, and semen samples
Serum samples were PCR-positive at all sampling times post inoculation. Blood-swab samples were positive at all but one sampling time post inoculation. Twenty-seven of 60 semen samples and 19 of 61 mouth-swab samples were positive (Table 2).
Table 2: Summary of polymerase chain reaction (PCR)* and ELISA† test results and occurrence of fever after intranasal inoculation of adult boars on Day 0 with 2.0 mL of porcine reproductive and respiratory syndrome virus (PRRSV) variant MNB04 containing 2 × 105 median tissue culture infective doses per mL
* Taqman PCR: a commercial kit (QIAamp DNA Blood BioRobot 9604 kit; Qiagen, Valencia, California) was used for RNA extractions, then reverse transcriptase-PCR was performed using a commercial kit (One Step RT-PCR kit; Qiagen). † Idexx HerdChek PRRS 2XR ELISA; Idexx Laboratories, Westbrook, Maine. ‡ Time after inoculation. § No. of animals positive for the parameter/no. of animals tested. One boar died during the study as a result of PRRSV infection. NA = not available (no test performed). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Virus was detected by PCR in serum and blood-swab samples 24 hours prior to detection in semen or mouth-swab samples. At ≥ 48 hours post inoculation, all boars sampled were detected positive by PCR testing of serum and blood swabs, whereas only four of 10 were detected positive by PCR testing of semen and three of 10 were detected positive by PCR testing of mouth swabs. In addition, there were no sampling times at which all boars tested were detected positive by PCR testing of semen or mouth-swab samples.
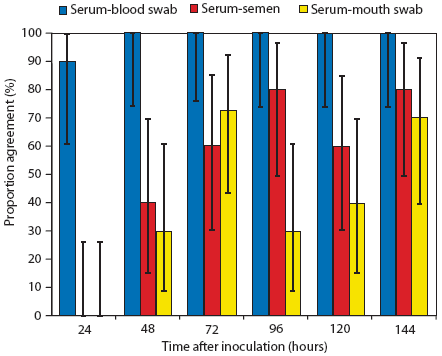
Evaluation of the proportion of agreement showed nearly perfect agreement between results of PCR testing of serum and blood swabs, with values ranging from 90% at 24 hours (90% CI: 61% – 99.5%) to 100% (90% CI: 74% – 100%) thereafter (Figure 3). Results of semen or mouth-swab PCR tests showed less consistent agreement with results of serum PCR tests throughout the study period (proportion of agreement varied between 0% and 80% depending on the sampling time) (Figure 3). Results of the McNemar‘s test for paired samples showed a significant difference between the results of serum and semen PCR tests at 24 hours and 48 hours (Figure 3). Results of serum and mouth-swab PCR tests were significantly different at 24 hours, 48 hours, 96 hours, and 120 hours (Figure 3).
Figure 3: Proportion of agreement and 90% confidence interval for results of polymerase chain reaction (PCR) tests performed on serum samples and blood swabs, serum and semen samples, and serum and mouth swabs collected from boars at various times after intranasal administration of 2.0 mL of inoculum containing porcine reproductive and respiratory syndrome virus variant MNB04 (3.2 × 105 median tissue culture infective doses per mL). The numbers of positive PCR results for each pair-wise combination of tests were compared at given sampling times using McNemar‘s test,12 with P < .10 considered statistically significant. Results of serum and semen PCR tests differed at 24 hours (P < .01) and 48 hours (P < .05). Results of serum and mouth-swab PCR tests differed at 24 hours (P < .01), 48 hours (P < .05), 96 hours (P < .05), and 120 hours (P < .05).
|
Survival analysis results indicated that median time to a PCR-positive test for serum samples and blood-swab samples was 24 hours (90% CI: 24 – 48 hours). Median time to a PCR-positive test was 72 hours (90% CI: 48 – 96 hours) for semen samples and 72 hours (90% CI: 72 – 120 hours) for mouth-swab samples.
A numerically larger amount of virus was detected by quantitative PCR in serum than in blood-swab samples. A numerically smaller amount of virus was detected in semen and mouth swabs than in either blood swabs or sera (Table 3). Concentration of virus in serum ranged from < 0.1 to 1291 IVP per mL, in blood swabs from < 0.1 to 158 IVP per mL, in semen from < 0.1 to 0.6 IVP per mL, and in mouth swabs from < 0.1 to 0.3 IVP per mL.
Table 3: Mean (± SD) concentration of porcine reproductive and respiratory syndrome virus (PRRSV) in samples* from adult boars (n = 10 per sampling time) by sample type and sampling time measured using quantitative polymerase chain reaction after intranasal inoculation with PRRSV variant MNB04†
* Quantitative PCR data were obtained by testing each sample that was initially PCR-positive (Table 2). A virus plaque assay10 was used as the standard and concentration of infectious virus particles (IVP) was reported. † 2.0 mL of inoculum was administered, containing 3.2 × 105 median tissue culture infective doses per mL. ‡ All positive samples read < 0.1. § One sample. NPS = no positive samples. |
|||||||||||||||||||||||||||||||||||||||
Serum ELISA
All boars tested at the start of the trial (Day 0) were serologically negative (S:P < 0.40) by PRRS ELISA. All boars tested on Day 23 post inoculation were serologically positive (S:P ≥0.40).
Rectal temperature
Fever was not associated with testing PCR-positive regardless of the sample type (P > .10).
Flinch rate
In each of the 20 cases when the blood-swab method was attempted from a vein near the tail head of the boar, the boar flinched. When the blood-swab method was performed using an ear vein while the boar was on the dummy ejaculating, a flinch was recorded in five of 52 procedures (9.6%).
Discussion
Most boar studs use PCR testing of semen as a monitoring tool for PRRSV, commonly testing a percentage of boar ejaculates due to cost constraints. Other studies have shown that PRRSV can be detected in semen during the first few days of infection.2,6,13 The availability and ease of semen sample collection cannot be disputed. However, it is clear that there is a significant lack of sensitivity in PCR testing of semen that delays the likelihood of detecting virus in the boar-stud population, thus putting sow herds that receive the semen at risk. It should not be surprising that this study showed that serum or blood-swab PCR results are more consistent and predictable than semen PCR results. In young pigs, virus can be detected in serum by virus isolation within 12 hours after infection.7 Virus was detected in serum from four of four boars at 1 day post infection and in serum earlier than in semen.6 In other studies involving small numbers of boars, virus was detected in serum before semen.4,5 In one study,14 no virus was detected in semen by virus isolation when nine boars were collected once per week through day 70 post inoculation. In other studies, semen was PRRSV-positive in four of 20 boars 4 to 14 days post infection,15 and in four of six boars, virus was detectable in semen for only 1 to 2 days.16 In a large study of 40 boars, only four boars were detected positive by semen PCR in the first 6 days after inoculation.3 One possible explanation for these variable results is that some strains of PRRSV enter the reproductive tract sooner than others and in varying quantities. Pathogenesis studies have shown that after initial viremia, PRRSV enters various tissues.17 In our study, there was a delay of 48 hours between detection of PCR-positive serum results and detection of PCR-positive semen results for the four boars with PCR-positive semen results. This confirms the delay between detection of PRRSV in serum and detection in semen. A breakdown of the blood-testis barrier must occur before virus enters the reproductive tract. In our experience, under field conditions, variation in detectable shedding of PRRSV in semen occurs during a PRRS outbreak in a boar stud. Virus strain differences may explain in part why this happens, and therefore may explain why more virus is detected in semen in some studies than in others. Other contributors to variation might be dose and animal susceptibility.
When random testing is performed in a boar stud, the likelihood of detecting PRRSV in any boar is low before an individual boar might be shedding detectable levels of virus in semen. The quantity of virus in the semen in this study was often at the minimum detection level (0.1 IVP per mL), which would mean that pooling of boar ejaculates (a commonly used testing procedure) further reduces the chances of detecting virus in a boar stud during the early stages of infection. Practically, every boar cannot be tested every day. The most common protocol is to sample boars the day they are collected. Due to the relatively high cost of PCR testing, a percentage of boars is likely to be sampled. The results of this study indicate that serum samples or blood swabs would contain virus sooner and in larger quantities than semen samples. Consequently, the blood-swab method would seem to be more appropriate for monitoring PRRS-negative boar studs, compared to the current industry practice of testing semen by PCR. It should be clarified that for semen distributed from PRRS-positive boar studs, semen PCR testing is still the most appropriate method. As a result of the low quantity of virus that can be found in semen, pooling of samples is not recommended.
There are obvious differences between serum sampling and the blood-swab method when monitoring boar studs. Animal restraint is not required with the blood-swab method. The boar can easily be sampled during ejaculation and little skill is required. The swabs used in this study hold approximately 0.1 to 0.2 uL of blood. If testing by serum, one needs to collect at least 1.0 mL of blood to have enough serum volume remaining, after centrifugation, to perform a PCR test. With the blood-swab method, saline provides most of the volume. It has been reported that inhibitors of RT-PCR occur in blood, including heme,18 leukocyte DNA,19 and immunoglobulin G.20 However, there did not appear to be inhibition in this study. This might be because of the short interval between collection and testing. Also, dilution of the blood in the saline, coupled with the short centrifugation step before extraction, may have made the inhibitory effects negligible.
While convenient, mouth-swab PCR does not appear to be an appropriate method for detecting PRRSV in boar studs. The PRRS virus has been identified in saliva up to 42 days post inoculation.21 Virus was found less frequently with the mouth-swab method than with any other method in this study. The quantity of virus in the positive mouth-swab samples was also commonly at the minimum detection level, so pooling of mouth-swab samples could not be justified.
Rectal temperatures did not appear to be a reliable predictor of virus detection in this study, which was similar to results obtained in a prior study.3
All but one blood-swab sample was positive in this study. As expected, virus was found in higher quantities in serum than in blood-swab samples due to the dilution effect of the blood-swab technique. First, whole blood remains in the sample in the blood-swab method and therefore dilutes PRRSV that might be present. This effect is relatively minor. Second, placing the saturated swab into 1 mL of saline solution dilutes virus that might be present. The amount of blood absorbed into the swab may also vary. The saline has the most significant diluting effect, and causes the resulting sample to be diluted 10-fold to 20-fold. Even though the agreement between serum and blood-swab samples was nearly perfect, strategies to reduce this dilution effect are being explored.
Only 9.6% of the boars flinched when the blood-swab method was performed in an ear vein during ejaculation. This seemed to be the ideal opportunity for sampling and also offers a great deal of safety for the person sampling. This is particularly true when a collection pen is used that is designed with the collector outside the collection pen.22
Pooling of samples is sought as an alternative to reduce cost. In order to maintain adequate sensitivity, dilution of a positive sample with negative samples should be minimal. Pooling is still an option with the blood-swab method that needs to be investigated, especially if the dilution effect can be reduced. Pooling of semen samples is not recommended if one is to expect reasonable sensitivity during early infection in a boar stud.
Taking into account convenience, safety, and sensitivity of detecting virus, the blood-swab method seems to be an appropriate monitoring method for PRRS-negative boar studs.
Implications
- The blood-swab collection method is a reliable alternative for sampling boars to test for PRRSV by PCR.
- Under the conditions of this study, PCR testing of blood-swabs is more sensitive than PCR testing of semen or mouth swabs.
- The presence of fever is not a reliable method to detect the presence of PRRSV in boars.
Acknowlegdement
This project was funded by the National Pork Board.
References
1. Robertson IB. Transmission of blue-eared pig disease. Vet Rec. 1992;130:478-479.
2. Yaeger MJ, Prieve T, Collins J, Christopher-Hennings J, Nelson E, Benfield D. Evidence for transmission of porcine reproductive and respiratory syndrome (PRRS) virus in boar semen. Swine Health Prod. 1993;1(5):7-9.
3. Reicks D, Mun~ oz-Zanzi C, Mengeling W, Christopher-Hennings J, Lager K, Polson D, Dee S, Rossow K. Detection of porcine reproductive and respiratory syndrome virus in semen and serum of boars during the first six days after inoculation. J Swine Health Prod. 2005;14:35-41.
4. Christopher-Hennings J, Holler LD, Benfield DA, Nelson EA. Detection and duration of porcine reproductive and respiratory syndrome virus in semen, serum, peripheral blood mononuclear cells and tissues from Yorkshire, Hampshire, and Landrace boars. J Vet Diagn Invest. 2001;13:133-142.
5. Christopher-Hennings J, Nelson EA, Nelson JK, Benfield DA. Effects of a modified-live virus vaccine against porcine reproductive and respiratory syndrome virus. Am J Vet Res. 1997;58:40-45.
6. Christopher-Hennings J, Nelson EA, Hines RJ, Nelson JK, Swenson SL, Zimmerman JJ, Chase CCL, Yaeger MJ, Benfield DA. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456-464.
7. Rossow KD, Collins JE, Goyal SM, Nelson EA, Christopher-Hennings J, Benfield DA. Pathogenesis of porcine reproductive and respiratory virus in gnotobiotic pigs. Vet Pathol. 1995;32:361-373.
8. Federation of Animal Science Societies. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. 1st rev ed. Savoy, Illinois: Federation of Animal Science Societies; 1999.
*9. Molitor TW, Tune KA, Shin J. Application of Taqman PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf. 1997;173-175.
10. Dulbecco R, Ginsberg HS. Virology. 2nd ed. Philadelphia, Pennsylvania: JB Lippincott; 1988:17-21.
11. Dohoo I, Martin W, Stryhn H. Screening and diagnostic tests. In: McPike M, ed. Veterinary Epidemiologic Research. AVC Inc. Charlottetown, Prince Edward Island, Canada. 2004:85-120.
12. Therneau T, Grambsch P. Modeling Survival Data. Extending the Cox Model. New York, New York: Springer; 2000.
13. Swenson SL, Hill HT, Zimmerman JJ, Evans LE, Wills RW, Yoon K-J, Schwartz KJ, Althouse GC, McGinley MJ, Brevik AK. Artificial insemination of gilts with porcine reproductive and respiratory syndrome virus-contaminated semen. Swine Health Prod. 1994;2:19-23.
14. Prieto C, Suarez P, Bautista JM, Sanchez R, Rillo SM, Simarro J, Solana A, Castro M. Semen changes after experimental infection with porcine reproductive and respiratory syndrome (PRRS) virus. Theriogenology. 1996;45:383-395.
15. Prieto C, Garcia C, Simarro I, Castro JM. Temporal shedding and persistence of porcine reproductive and respiratory syndrome in boars. Vet Rec. 2004;154:824-827.
16. Wasilk A, Callahan J, Christopher-Hennings J, Gay T, Fang Y, Dammen M, Reos M, Torremorell M, Polson D, Mellencamp M, Nelson E, Nelson W. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42:4453-4461.
17. Christopher-Hennings J, Nelson E, Nelson J, Rossow K, Shivers J, Yaeger M, Chase C, Garduno R, Collins J, Benfield D. Identification of porcine reproductive and respiratory syndrome virus in semen and tissues of vasectomized and non-vasectomized boars. Vet Path. 1998;35:260-267.
18. Akane A, Matsubara K, Nakamura H, Takahashi S, Kimura K. Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification. Forensic Sci. 1994;39:362-372.
19. Morata P, Queipo-Ortuno MI, de Dios Colmenero J. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J Clin Microbiol. 1998;36:2443-2446.
20. Al Soud WA, Radstrom P. Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol. 2001;39:485-493.
21. Wills RW, Zimmerman JJ, Yoon K-J, Swenson SL, Hoffman LJ, McGinley MJ, Hill HT, Platt KB. Porcine reproductive and respiratory syndrome virus: route of excretion. Vet Microbiol. 1997;57:69-81.
22. Levis D, Reicks D. Assessment of sexual behavior and effect of semen collection pen design and sexual stimulation of boars on behavior and sperm output – a review. Theriogenology 2005;63:630-642.
* Non-refereed reference.