Original research |
Peer reviewed |
Alternatives to the use of antimicrobial feed additives in nursery diets: A pilot study
Alternativas al uso de aditivos antimicrobianos en las dietas de destete: Un estudio piloto
Alternatives à l’usage d’additifs antimicrobiens dans le régime alimentaire des pouponnières: Une étude pilote
Darryl Ragland, DVM, PhD; Jessica L. Schneider, RVT; Sandra F. Amass, DVM, PhD, Diplomate ABVP; Michael A. Hill, BVetMed, MS, PhD, MRCVS
Department of Veterinary Clinical Sciences, Purdue University School of Veterinary Medicine, West Lafayette, Indiana. Corresponding author: Dr Darryl Ragland, Purdue University, VCS/LYNN, 625 Harrison Street, West Lafayette, IN 47907-2026; Tel: 765-494-1209; Fax: 765-496-2608.
Cite as: Ragland D, Schneider JL, Amass SF, et al. Alternatives to the use of antimicrobial feed additives in nursery diets: A pilot study. J Swine Health Prod. 2006;14(2):82-88.
Also available as a PDF.
SummaryObjective: To evaluate the effect of inorganic minerals and probiotics on pig performance and antimicrobial resistance. Materials and methods: Ninety-six crossbred pigs 17 to 20 days old were randomly assigned to six experimental diets. A non-medicated basal diet was supplemented with carbadox (55.12 g per tonne), copper sulfate (192.40 g copper per tonne), zinc oxide (2712.68 g zinc per tonne), or the probiotic feed additive BioPlus2B (Chr Hansen Inc, Milwaukee, Wisconsin; 1.1 x 106 or 1.3 x 106 spores per gram of feed). Pigs were weighed weekly and feed consumption was monitored to assess performance. Rectal swabs were collected for isolation of enterococci to evaluate antimicrobial resistance. Isolates were screened for resistance to vancomycin, and the broth microdilution procedure was used to determine minimum inhibitory concentration breakpoints. Results: The zinc oxide diet promoted the heaviest bodyweights and greatest feed consumption (P < .05). Feed:gain was similar for pigs fed the diets supplemented with carbadox, BioPlus2B at 1.3 x 106 spores per gram, copper sulfate, and zinc oxide. Enterococcal isolates were highly susceptible to vancomycin at the start and end of the study. Implications: Zinc oxide may sustain acceptable pig performance as the sole growth promotant in nursery diets. BioPlus2B in nursery pig diets did not enhance growth or feed utilization. In this study, the young pig did not represent a significant reservoir of vancomycin-resistant enterococci. | ResumenObjetivo: Evaluar el efecto de los minerales inorgánicos y probióticos en el desempeño de los cerdos y la resistencia antimicrobiana. Materiales y métodos: Noventa y seis cerdos comerciales de 17 a 20 días de edad se asignaron al azar a seis dietas experimentales. Una dieta basal no medicada se suplementó con carbadox (55.12 g por tonelada), sulfato de cobre (192.40 g de cobre por tonelada), óxido de zinc (2712.68 g de zinc por tonelada), o con el probiótico para el alimento BioPlus2B (Chr Hansen Inc, Milwaukee, Wisconsin) a 1.1 x 106 ó 1.3 x 106 esporas por gramo de alimento. Los cerdos se pesaron cada semana y se monitoreo el consumo de alimento para evaluar el comportamiento productivo. Se tomaron hisopos réctales para el aislamiento de enterococci y para evaluar la resistencia antimicrobiana. Los aislamientos fueron evaluados para su resistencia a la vancomicina y la técnica de microdilución en caldo se utilizó para determinar los puntos de corte de la concentración mínima inhibitoria. Resultados: La dieta que contenía el óxido de zinc logró los mejores pesos corporales y el mejor consuno de alimento (P < .05). La ganancia de alimento fue similar en los cerdos cuya dieta fue suplementada con carbadox, BioPlus2B a 1.3 x 106 esporas por gramo, sulfato de cobre, y óxido de zinc. Los aislamientos de enterococos fueron altamente susceptibles a la vancomicina al principio y al final del estudio. Implicaciones: El óxido de zinc puede mantener un desarrollo aceptable del cerdo si se utiliza como el único promotor de crecimiento en las dietas del destete. Se requiere más investigación para determinar las circunstancias donde el BioPlus2B puede ser utilizado de la manera más efectiva para promover el crecimiento del cerdo y la utilización del alimento. En este estudio, el cerdo joven no representó un reservorio significativo de enterococci resistente a la vancomicina. | ResuméObjectif: Évaluer l’effet des minéraux inorganiques et des probiotiques sur le rendement des porcs et sur la résistance aux antimicrobiens. Matériel et méthodes: Quatre-vingt-seize porcelets croisés âgés de 17 à 20 jours ont fait l’objet d’une répartition aléatoire à six régimes expérimentaux, soit un régime de base non médicamenté avec un supplément de carbadox (55.12 g par tonne), de sulfate de cuivre (192.40 g de cuivre par tonne), d’oxyde de zinc (2712.68 g de zinc par tonne), ou d’un supplément alimentaire probiotique, le BioPlus2B (Chr Hansen Inc, Milwaukee, Wisconsin), à raison de 1.1 x 106 ou de 1.3 x 106 spores par gramme de nourriture. On a pesé les porcelets chaque semaine et on a contrôlé la consommation de nourriture afin d’évaluer le rendement. On a aussi procédé à la collecte d’écouvil-lonnages rectaux en vue de l’isolation d’entérocoques pour évaluer la résistance aux antimicrobiens. Les isolats ont été testés pour la résistance à la vancomycine et on a eu recours à la technique de la microdilution en milieu liquide afin de déterminer les points de rupture minimums de la concentration inhibitrice. Résultats: Le régime à base d’oxyde de zinc a donné lieu à la plus grande prise de poids et à la meilleure consommation de nour-riture (P < .05). La proportion nourriture:rende-ment était semblable pour les porcelets recevant le régime contenant les supplé-ments de carbadox, de BioPlus2B à raison de 1.3 x 106 spores par gramme, de sulfate de cuivre, et d’oxyde de zinc. Les isolats d’entérocoques étaient très sensibles à la vancomycine tant au début qu’à la fin de l’étude. Implications: L’oxyde de zinc pourrait offrir un rendement suffisant à titre d’unique stimulateur de croissance dans le régime alimentaire des pouponnières. L’ajout de BioPlus2B à la nourriture des porcelets n’a pas amélioré la croissance ni la consommation de nourriture. Dans le cadre de cette étude, le jeune porc ne constituait pas un réservoir significatif d’entérocoques résistants à la vancomycine. |
Keywords: swine, inorganic
minerals, probiotics, antimicrobial resistance, enterococcus
Search the AASV web site
for pages with similar keywords.
Received: February
24, 2004
Accepted: July
5, 2004
The use of feed-grade antimicrobials at subtherapeutic levels for growth promotion has evolved into a highly controversial issue, subjecting animal agriculture to increasing scrutiny. This use of antimicrobials is being vigorously challenged by human medical organizations and special interest groups, because they perceive that it has encouraged the emergence of antimicrobial-resistant bacteria that are being disseminated to human beings through the food chain.1 Because a conclusive link between the practice of antimicrobial growth promotion in animals and transfer of resistant bacteria to human beings has yet to be demonstrated, the validity of such a conclusion is tenuous.2 However, in the absence of scientific evidence, the vigor with which this issue is being pursued has prompted federal legislation that, if approved, would restrict the availability of antimicrobials for growth promotion. In Denmark, a ban on subtherapeutic use of antimicrobials has resulted in a near doubling of the total usage of antimicrobials for therapeutic treatment of animals that have succumbed to infectious diseases.3 Therefore, a ban on subtherapeutic antimicrobial use in swine feeding programs could exert a profoundly negative effect on the health and welfare of pigs in the United States.
The controversy surrounding antimicrobial growth promotion and demands for an immediate ban of the practice have prompted interest in alternatives to the use of antimicrobials in swine feeding programs. A plethora of products described as antimicrobial alternatives have been available for years, and some represent integral components of swine nutritional programs for nursery pigs.4,5 However, there has been reluctance to adopt these products in swine feeding programs as sole replacements to antimicrobial growth promoters because questions persist about their ability to effectively enhance performance and suppress disease. There is also a lack of knowledge related to antimicrobial resistance patterns associated with ingestion of compounds described as antimicrobial alternatives. Therefore, this pilot study was undertaken with the intent of achieving two objectives related to the use of antimicrobial alternatives in swine feeding programs. The first objective was to evaluate pig performance in response to inorganic mineral supplementation and probiotic supplementation of swine diets. The second objective was to determine if consumption of inorganic minerals and probiotic feed supplements influence development of vancomycin resistance in enterococci.
Materials and methods
Experimental design
A 35-day growth assay was used to evaluate the effect of inorganic mineral or probiotic supplementation of nursery diets on pig performance. Pigs were assigned to treatments on entering the nursery (Day 0). The experimental design consisted of a randomized complete block design with a total of four replicate blocks. Pen constituted the experimental unit and six dietary treatments were used (Table 1).
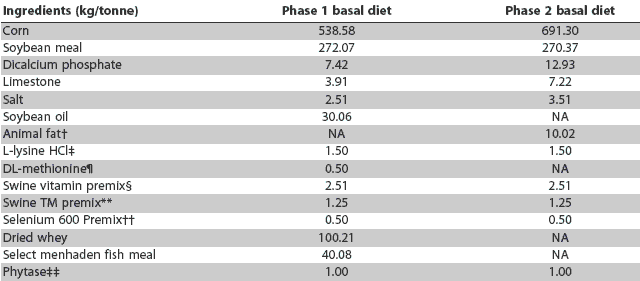
Table 1: Nursery diet composition for pigs fed either a basal diet or the same diet supplemented with a probiotic, carbadox, copper sulfate, or zinc oxide at the expense of corn*
* Phase 1 diets were fed Days 0 to 21 and Phase 2 diets Days 21 to 35. BioPlus2B (Chr Hansen Inc, Milwaukee, Wisconsin) was added to the basal diet at 0.50 kg/tonne (1.1 x 106 spores/g of feed) or 0.60 kg/tonne (1.3 x 106 spores/g of feed). Carbadox was added to the basal diet at 2.51 kg/tonne. Copper sulfate (5.2%) was added to the basal diet at 0.75 kg/tonne (192.40 g copper/tonne). Zinc oxide (72%) was added to the basal diet at 3.76 kg/tonne (2712.68 g zinc/tonne). † Choice white grease. ‡ 78.5% L-lysine. ¶ 99% methionine. § Supplied per kg of Phase 1 complete diet: vitamin A, 6105 IU; vitamin D, 611 IU; vitamin E, 44 IU; vitamin B12, 40 mg; menadione, 2.0 mg; riboflavin, 7.2 mg; d-pantothenic acid, 22.2 mg; niacin, 44 mg. Supplied per kg of Phase 2 complete diet: vitamin A, 5990 IU; vitamin D, 599 IU; vitamin E, 43.6 IU; vitamin B12, 30 mg; menadione, 2.0 mg; riboflavin, 7.0 mg; d-pantothenic acid, 21.8 mg; niacin, 43.6 mg. ** Supplied per kg of Phase 1 complete diet: copper, 11.2 mg; iodine, 0.42 mg; iron, 120 mg; manganese, 14.9 mg; zinc, 120 mg. Supplied per kg of Phase 2 complete diet: copper, 11.4 mg; iodine, 0.42 mg; iron, 122.6 mg; manganese, 15.2 mg; zinc, 122.6 mg. †† Selenium, 0.301 g/tonne. ‡‡ Natuphos 600 (BASF Animal Nutrition, Florham Park, New Jersey), 600 FTU phytase/g. NA = not applicable. |
Pigs were weighed weekly and a feed record was maintained to monitor feed additions. Feeder weights were obtained on Day 21 (when the change was made from Phase 1 to Phase 2 diets), and on Day 35 (when the growth aspect of the study was terminated).
Pigs and housing
Ninety-six 17- to 20-day old crossbred pigs were assigned to the study, with equal numbers of barrows and gilts, weighing 5.94 to 5.96 kg on Day 0. Pigs selected for the study had not been treated with therapeutic antimicrobials and the dams of these pigs had not been treated with therapeutic antimicrobials during lactation. Pigs that developed medical conditions requiring antimicrobial therapy were withdrawn from the study and moved to another location for treatment to avoid potential introduction of bias with regard to measurement of antimicrobial resistance. The animal use protocol for the study was approved by Purdue University’s Institutional Animal Care and Use Committee.
Pigs were housed at the Purdue University Animal Sciences Research and Education Center Swine Unit (West Lafayette, Indiana), four per pen in an environmentally controlled nursery in 1.98-m2 pens with plastic-coated floors. Each pen was fitted with plywood partitions to prevent fecal contamination from adjacent pens.
Diets
Diet composition was representative of standard nursery diet formulations used by the Purdue University Animal Sciences Research and Education Center Swine Unit. An unmedicated basal diet (negative control) was formulated that met or exceeded NRC recommendations for nursery-age pigs.6 A medicated diet (positive control) was formulated by supplementing the basal diet formulation with carbadox at 55.12 g per tonne.
A diet containing pharmacological levels of copper was formulated by supplementing the basal diet formulation with copper sulfate (192.40 g copper per tonne), hereafter referred to as CuSO4. A diet containing pharmacological levels of zinc was formulated by supplementing the basal diet formulation with zinc oxide (2712.68 g zinc per tonne), hereafter referred to as ZnO. The probiotic BioPlus2B (Chr Hansen Inc, Milwaukee, Wisconsin) was added to the basal diet formulation at two concentrations, 1.1 x 106 or 1.3 x 106 spores per g of feed, hereafter referred to as BP1 and BP2, respectively. BioPlus2B contains spores of Bacillus licheniformis and Bacillus subtilis. The level of 1.1 x 106 spores per g represents the manufacturer’s recommended inclusion rate for swine diets in the United States, and the level of 1.3 x 106 spores per g represents the authorized inclusion rate for swine diets in the European Union.
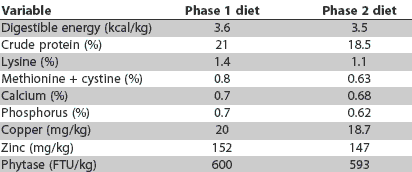
Feed additives were added to the basal diet formulation at the expense of corn. The experimental diets were fed in meal form and were isonitrogenous and isocaloric. A phase-feeding protocol was used: the first nursery diet (Phase 1) was fed for the initial 21 days of the study and the second nursery diet (Phase 2) for the last 14 days. Diet compositions of the Phase 1 and Phase 2 basal diets are provided in Table 1, and calculated nutrient compositions in Table 2. The Phase 1 diets contained soybean oil, DL-methionine, dried whey, and select menhaden fish meal, whereas these products were eliminated from the Phase 2 diet formulations. Animal fat was added to the Phase 2 diet. The diets were prepared and bagged by the staff at the Purdue University Feed Mill.
Table 2: Calculated nutrient composition of the Phase 1 and Phase 2 basal nursery diets described in Table 1
|
Microbiology
Enterococci were cultured from rectal swabs from pigs and served as the sentinel organism to evaluate development of antimicrobial resistance in response to consumption of the experimental diets. Rectal swabs collected on Days 0, 21, and 35 were immersed in a commercially prepared solution (Cary-Blair transport media; Becton Dickinson, Franklin Lakes, New Jersey) and chilled on ice packs upon collection prior to transport to the laboratory. Swabs were homogenized in 0.3 mL of sterile water and a 10-mL sample streaked onto bile esculin agar plates to obtain individual colonies. Bile esculin agar plates were incubated aerobically at 35°C for approximately 24 hours. Colonies that hydrolyzed esculin in the agar were transferred to blood agar plates and incubated aerobically at 35°C for 24 hours to obtain pure colonies. As a presumptive test, the blood agar isolates were subjected to the catalase test, and isolates that demonstrated a weak reaction or no reaction were saved for definitive identification. Enterococci were identified using the AP120E biochemical analysis kit (bioMerieux, Durham, North Carolina), a system of enzymatic and fermentation reactions used for bacterial identification. The broth microdilution assay was used to determine susceptibility of enterococcus isolates to the glycopeptide antibiotic vancomycin hydrochloride (Sigma-Aldrich, St Louis, Missouri). This assay was performed according to the methods described by the National Committee for Clinical Laboratory Standards.7 Concentrations of vancomycin used to screen the enterococcus isolates for resistance were 0, 2, 4, 8, 16, 32, 64, 128, and 256 mg per L, which were identical to concentrations used in the microbiological laboratory of a local human medical center (Home Hospital, Lafayette, Indiana) as part of their antimicrobial resistance surveillance program. Minimum inhibitory concentrations (MIC) >= 32 mg per L were considered evidence of resistance to vancomycin.7
Calculations and statistical analysis
Least squares means for bodyweight, average daily gain (ADG), feed intake, feed efficiency, and MIC were calculated in SAS (SAS Institute, Cary, North Carolina) and subjected to the general linear models (GLM) procedure. The MIC results for the rectal samples collected on Days 21 and 35 were pooled for analysis. The least significant difference test was used as the mean separation procedure. For all tests, P < .05 was considered statistically significant.
Results
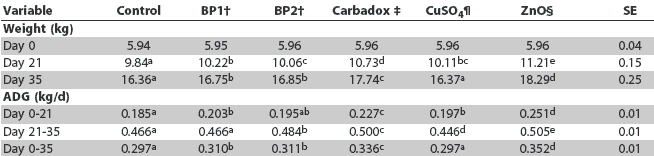
Bodyweight and average daily gain data are reported in Table 3, and feed consumption and feed efficiency data in Table 4. Pigs fed the diet supplemented with ZnO had the heaviest bodyweights on Days 21 and 35 (P < .05) and experienced the greatest ADG for the initial 21 days of the study (P < .05), compared to the other dietary treatments. Average daily gain was similar for pigs fed the ZnO and carbadox diets for the last 14 days of the study (P > .05), and was greatest for pigs fed the ZnO diet for the 35-day period (P < .05). On Day 21, bodyweights of pigs fed the BioPlus2B diets exceeded bodyweights of pigs fed the basal diet (P < .05), and were similar to bodyweights of pigs fed the CuSO4 diet (P > .05). On Day 35, pigs fed both BioPlus2B diets were heavier (P < .05) than pigs fed the basal and CuSO4 diets. In pigs fed the BioPlus2B diets, ADG was similar (P > .05) during the initial 21 days of the study and for the 35-day period. The ADG of pigs fed the BP2 diet exceeded (P < .05) that of pigs fed the BP1 diet during the last 14 days of the study. At 35 days, bodyweights of pigs fed the basal and CuSO4 diets were similar (P > .05) and were the lowest among all dietary treatments. Pigs fed the CuSO4 diet achieved heavier (P < .05) bodyweights on Day 21 compared to pigs fed the basal diet; however, pigs fed the basal diet experienced greater (P < .05) ADG than pigs fed the CuSO4 diet between Days 21 and 35. The ADG was similar (P > .05) for pigs fed the basal and CuSO4 diets over the 35-day study period.
Table 3: Least squares means of body weight and average daily gain (ADG) of nursery pigs fed basal diets (control) or the same diets supplemented with Bioplus2B (a probiotic), carbadox, copper sulfate, or zinc oxide*
* Phase 1 diets were fed upon entering the nursery (Days 0 to 21) and Phase 2 diets Days 21 to 35. Supplemented diets were manufactured at the expense of corn in the basal diets. BP1 = BioPlus2B, a probiotic (Chr Hansen Inc, Milwaukee, Wisconsin), at 1.1 x 106 spores/g of feed; BP2 = BioPlus2B at 1.3 x 106 spores/g of feed. Carbadox at 55.12 g/tonne. ¶ CuSO4 = copper sulphate at 192.4 g copper/tonne. § ZnO = zinc oxide at 2712.68 g zinc/tonne. abcde Values with different superscripts within a row are statistically different (least significant difference test; P < .05). |
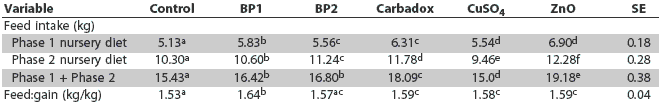
Table 4: Least squares means of feed consumption and feed efficiency of nursery pigs fed basal diets (control) or the same diets supplemented with BioPlus2B (a probiotic), carbadox, copper sulfate, or zinc oxide*
* Diets described in Table 3. abcdef Values with different superscripts within a row are statistically different (least significant difference test; P < .05). |
Overall, pigs fed the ZnO diet had significantly higher (P < .05) feed intakes compared to pigs fed the other dietary treatments. Feed:gain ratio was highest for pigs fed BP1 diet and lowest for pigs fed the basal diet. Feed:gain for pigs fed the BP2 diet was similar (P > .05) to that of pigs fed the basal diet, and feed:gain was similar for pigs fed the BP2, carbadox, CuSO4, and ZnO diets (P > .05).
Mean MIC of enterococcal isolates recovered on Day 0 and after consumption of the experimental diets on Days 21 and 35 (combined for analysis) are shown in Table 5. The MIC results for enterococci recovered on Days 21 and 35 were combined due to poor recovery of enterococci from pigs fed the BP1, CuSO4, and ZnO diets. Enterococci were identified to the species level and in rank order, isolates consisted of Enterococcus faecium (69), Enterococcus faecalis (33), Enterococcus avium (27), Enterococcus durans (20), and Enterococcus gallinarum (1), respectively. Except for enterococci isolated from pigs assigned the BP2 diet, mean MICs on Day 0 were similar (P > .05). Even though MICs of isolates from pigs assigned the BP2 diet differed significantly from MICs of isolates from pigs fed the other diets, they still resided in the susceptible range for vancomycin. Mean MIC for the pooled Day 21 and Day 35 samples were similar (P > .05) for enterococci from pigs fed the BP1, BP2, CuSO4, and ZnO diets. However, the MICs of pigs fed the basal and carbadox diets were greater (P < .05) than those for pigs fed the other experimental treatments. The MICs for the basal and carbadox diets both resided in the susceptible range for vancomycin. A total of nine pigs were removed from the study for welfare reasons. Seven pigs were removed due to exudative epidermitis that warranted antimicrobial therapy: one on the basal diet, one on the BP1 diet, one on the CuSO4 diet, and four on the ZnO diet. Two pigs on the CuSO4 diet were removed because they failed to make productive weight gains during the initial 3 weeks of the study.
Table 5: Least squares means of prefeeding and postfeeding MICs, determined by broth microdilution assay, of enterococci isolated from rectal swabs of nursery pigs fed basal diets (control) or the same diets supplemented with BioPlus2B (a probiotic), carbadox, copper sulfate, or zinc oxide*
* Diets described in Table 3. Prefeeding samples were collected when pigs entered the nursery (Day 0) at 17 to 20 days of age. Postfeeding samples were collected Day 21 (when Phase 1 diets were replaced by Phase 2 diets) and Day 35. abc Values with different superscripts within a row are statistically different (least significant difference test; P < .05). |
Discussion
Alternatives to the use of antimicrobials for growth promotion in animal production continue to evolve, and some that have shown promise for reducing antimicrobial usage in swine production include inorganic minerals and probiotics. In the present study, the diet supplemented with ZnO yielded the best overall performance. It was anticipated that the diet supplemented with carbadox would yield the best pig performance, since antimicrobial feed additives historically have been shown to enhance growth and feed efficiency to a much greater extent than nonantimicrobial feed additives.8 Our results were similar to those of other investigators who have reported that performance of nursery pigs fed ZnO matched or exceeded performance of pigs fed carbadox.9 Moreover, other studies have demonstrated that enhancement of growth and feed efficiency of nursery-age pigs is a consistent property of ZnO supplementation.10,11 The exact mechanism of action of ZnO is still unknown, but stabilization of the enteric flora has been proposed.12 In addition, systemic and local enteric effects have been proposed to account for the growth-promoting effects of zinc when fed at pharmacologic levels encompassing 2000 to 3000 ppm (ie, 2000 to 3000 g per tonne).13 On the basis of the feed intake data in our study, enhanced feed intake appears to account for part of the significant improvement in pig performance. The diet supplemented with CuSO4 yielded poor responses in growth and feed efficiency. Copper sulfate has been described as an alternative to the use of antimicrobials for growth promotion in nursery diets and is described as having an antimicrobial effect,6 but the enhancement of pig performance does not appear to be as potent as that for ZnO.10,11 Numerous studies have demonstrated a growth-promoting effect when copper is fed at pharmacological levels encompassing 100 to 250 ppm (ie, 100 to 250 g per tonne);14-16 however, the results obtained in our study are consistent with negative growth and feed-intake responses reported in a series of feeding trials with nursery pigs.17 Compared to the feed intakes achieved by pigs fed the ZnO-supplemented diet in this study, it appears that reduced feed intake is partly responsible for the poor growth response obtained with the diet supplemented with CuSO4.
In a compilation of studies with probiotics, investigators have recognized an overall benefit to feeding these products, but inconsistencies have prompted caution with respect to their use on a larger scale.8 Investigators in Europe found that probiotics consisting of bacillus spores had favorable effects on pig performance as well as control of enteric pathogens.18,19 In our study, growth of pigs fed the diets supplemented with BioPlus2B lagged behind that of pigs consuming the diets supplemented with ZnO or carbadox.
Enterococci were selected for monitoring antimicrobial resistance because they are alleged to foster dissemination, from animals to humans, of genetic elements that facilitate resistance to vancomycin.20 The isolates collected on Days 21 and 35 were combined for analysis due to poor recovery of enterococci from pigs fed the BP1, CuSO4, and ZnO diets. We presume that the dietary treatments altered the enteric flora such that our ability to isolate enterococci from the fecal samples was reduced. Enrichment of samples with nutrient broth prior to plating on bile esculin agar will be employed in future studies to overcome this problem. Vancomycin was selected to screen the isolates for resistance to assess the degree of vancomycin resistance associated with enterococci obtained from nursery pigs. Vancomycin use in food animals in the United States is not permitted under any circumstances.21 Therefore, development of vancomycin resistance in enterococci originating from food animals reflects a mechanism exclusive of selective pressure from vancomycin use. In our study, we inferred that nursery-age pigs do not represent a significant reservoir of vancomycin-resistant enterococci. Minimum inhibitory concentrations corresponding to >= 32 mg per L were considered resistant, and the MIC for all dietary treatments clustered around 2 mg per L. We recovered a heterogeneous sample of enterococci, and E faecium constituted the predominant species (46%). Enterococcus faecium is of concern in the human health arena because it is recognized as possessing genetic traits that allow it to resist the action of vancomycin and other antimicrobials.22 However, heterogeneity of the isolates did not appear to confound the susceptibility assays. Considering the predominance of E faecium in the sample pool and clustering of MIC for all treatments around 2 mg per L, we concluded that the isolates in our study lacked the genetic traits that would promote high-level resistance to vancomycin. The MIC of 32 mg per L that was considered evidence of resistance to vancomycin was based on the standard established for humans, because an MIC for vancomycin in animals does not exist.6 The vancomycin concentrations mirrored those used as part of the antimicrobial resistance surveillance program in the microbiological laboratory of the local human medical center and were 0, 2, 4, 8, 16, 32, 64, 128, and 256 mg per L. In retrospect, concentrations corresponding to 0.5, 1.0, and 3.0 mg per L vancomycin should have been included in the panel to better characterize the susceptibility of the isolates. The diets containing ZnO, CuSO4, or BioPlus2B did not promote an increase in MIC to vancomycin. However, isolates from pigs fed the basal and carbadox diets did exhibit a slight increase in MIC to vancomycin. Although this increase in MIC was inconsequential, this observation illustrates the complexity of the phenomenon of antimicrobial resistance and raises further questions regarding the influence of antimicrobial use on the expression of antimicrobial resistance. Carbadox was the only antimicrobial to which pigs were exposed in our study, and therapeutic antimicrobials were not used to treat any pigs enrolled in the study.
Nine pigs had to be removed from the study: seven developed exudative epidermitis that warranted antimicrobial therapy, and two failed to thrive after the initial 3 weeks of the study. First-parity sows comprised the majority of females in the farrowing group from which the pigs used in the study were derived. The prevalence of exudative epidermitis was attributed to inadequate immunocompetence of the parity-one females in the farrowing group to Staphylococcus hyicus. All affected pigs were relocated to another room, treated with therapeutic antimicrobials, and never returned to the nursery where the study was conducted. Also, the pens were separated by plywood partitions to prevent fecal contamination of adjacent pens by pigs that were fed different diets. Therefore, the slight increase in MIC for the basal diet is difficult to interpret, considering the lack of exposure of pigs to vancomycin. However, emergence of acquired resistance has been described in human beings that were not subjected to heavy consumption of antimicrobials, indicating that selective pressure is not always necessary to promote development of antimicrobial resistance.23 It is within this perspective that the MIC for pigs fed the basal diet might increase over time without exposure to antimicrobials.
The results of our study indicate that nonantimicrobial feed additives may represent a viable replacement for antimicrobial feed additives in nursery diet formulations. Furthermore, our results demonstrate that BioPlus2B, carbadox, and the inorganic forms of copper and zinc fed at pharmacologic levels do not promote vancomycin resistance in enterococci.
Implications
- Zinc oxide may sustain acceptable pig performance as the sole growth promotant in nursery diets.
- Under the conditions of this study, BioPlus2B in nursery pig diets does not enhance growth or feed utilization.
- In this study, the young pig did not represent a significant reservoir of vancomycin-resistant enterococci.
Acknowledgment
Financial support for completion of the research project was provided by The National Pork Board and is gratefully acknowledged.
References
1. Willis C. Antibiotics in the food chain: their impact on the consumer. Rev Med Microbiol. 2000;11:153-160.
2. Elder HA, Roy I, Lehman S, Phillips RL, Kass EH. Human studies to measure the effect of antibiotic residues. Vet Human Toxicol. 1993;35:31-36.
3. Casewell M, Friis C, Marco E, McMullin P, Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. 2003;52:159-161.
*4. Pettigrew JE. Alternative products: are there any silver bullets? Proc AASV. Orlando, Florida. 2003;439-441.
5. Holm A, Poulsen HD. Zinc oxide in treating E. coli diarrhea in pigs after weaning. The Compendium. 1996;18:S26-S29,S48.
6. National Research Council. 1998. Nutrient Requirements of Swine. National Research Council, 10th revised ed. Washington, DC: National Academy Press.
7. National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard. 6th ed. Wayne, Pennsylvania: NCCLS; January 2003. Document M7-A6.
8. Cromwell GL. Antimicrobial and promicrobial agents. In: Lewis AJ, Southern LL, eds. Swine Nutrition. 2nd ed. New York: CRC Press. 2001:401-426.
9. Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, Lewis AJ, Miller PS, Shurson GC, Veum TL. Effect of pharmacological concentrations of zinc oxide with or without inclusion of an antibacterial agent on nursery pig performance. J Anim Sci. 2001;79:934-941.
10. Hill GM, Cromwell GL, Crenshaw TD, Dove CR, Ewan RC, Knabe DA, Lewis AJ, Libal GW, Mahan DC, Shurson GC, Southern LL, Veum TL. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J Anim Sci. 2000;78:1010-1016.
11. Smith JW, Tokach MD, Goodband RD, Nelssen JL, Richert BT. Effects of the interrelationship between zinc oxide and copper sulfate on growth performance of early-weaned pigs. J Anim Sci. 1997;75:1861-1866.
12. Katouli M, Jensen-Waern M, Wallgren P, Mollby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol. 1999;87:564-573.
13. Case CL, Carlson MS. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J Anim Sci. 2002;80:1917-1924.
14. Armstrong TA, Cook DR, Ward MM, Williams CM, Spears JW. Effect of dietary copper source (cupric citrate and cupric sulfate) and concentration on growth performance and fecal copper excretion in weanling pigs. J Anim Sci. 2004;82:1234-1240.
15. Veum TL, Carlson MS, Wu CW, Bollinger DW, Ellersieck MR. Copper proteinate in weanling pig diets for enhancing growth performance and reducing fecal copper excretion compared with copper sulfate. J Anim Sci. 2004;82:1062-1070.
16. Apgar GA, Kornegay ET, Lindemann MD, Notter DR. Evaluation of copper sulfate and a copper lysine complex as growth promoters for weanling swine. J Anim Sci. 1995;73:2640-2646.
17. Stansbury WF, Tribble LF, Orr DE Jr. Effect of chelated copper sources on performance of nursery and growing pigs. J Anim Sci. 1990;68:1318-1322.
18. Kyriakis SC, Tsiloyiannis VK, Vlemmas J, Sarris K, Tsinas AC, Alexopoulos C, Jansegers L. The effect of probiotic LSP 122 on the control of post-weaning diarrhoea syndrome of piglets. Res Vet Sci. 1999;67:223-228.
19. Zani JL, da Cruz FW, dos Santos AF, Gil-Turnes C. Efficacy of CenBiot on the control of diarrhoea and feed efficiency in pigs. J Appl Microbiol. 1998;84:68-71.
20. Robredo B, Singh KV, Baquero F, Murray BE, Torres C. Vancomycin-resistant enterococci isolated from animals and food. Int J Food Microbiol. 2000;54:197-204.
21. Extralabel drug use in animals. Drugs prohibited for extralabel use in animals. Rockville, Maryland: US Department of Health and Human Services. Food and Drug Administration. US Code of Federal Regulations; April 1, 2002. 21CFR530.41. Available at: http://www.gpoaccess.gov/cfr/index.html. Accessed January 4, 2006.
22. Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239-249.
23. Bartoloni A, Bartalesi F, Mantella A, Dell’Amico E, Roselli M, Strohmeyer M, Barahona HG, Barron VP, Paradisi F, Rossolini GM. High prevalence of acquired antimicrobial resistance unrelated to heavy antimicrobial consumption. J Infect Dis. 2004;189:1291-1294.
* Non-refereed reference.