Original research |
Peer reviewed |
Evaluation of three strategies for insect control on a commercial swine farm
Evaluación de tres estrategias para el control de insectos en una granja comercial porcina
Évaluation de trois stratégies de lutte contre les insectes dans un élevage de porcs commercial
J. A. Schurrer, BA; S. A. Dee, DVM, MS, PhD, Diplomate ACVM; R. D. Moon, PhD; J. Deen, DVM, PhD, Diplomate ABVP; C. Pijoan, DVM, PhD
JAS, SAD, JD, CP: Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, St Paul, Minnesota. RDM: Department of Entomology, University of Minnesota College of Agriculture, St Paul, Minnesota. Corresponding author: Dr S. A. Dee, Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, Room 385 C, 1988 Fitch Avenue, St Paul, MN 55108; Tel: 612-625-4786; Fax: 612-625-1210; E-mail: deexx004@umn.edu.
Cite as: Schurrer JA, Dee SA, Moon RD, et al. Evaluation of three strategies for insect control on a commercial swine farm. J Swine Health Prod. 2006;14(2):76-81.
Also available as a PDF.
SummaryObjective: To assess the efficacy of three intervention strategies for control of insects on a commercial swine farm. Methods: The study was conducted on a commercial finishing facility that contained four rooms. Three strategies (treatments) were evaluated: insect screening of sidewall inlets, application of pyrethroid-based insecticide to the walls and floors of the building, the combination of inlet screens and insecticide, and no treatment (negative control). Each room was assigned a treatment for a 2-week sampling period. Specific parameters measured biweekly included numbers of flies and mosquitoes per room, number of insect bites per pig, number of fly spots per room, and level of carbon dioxide, temperature, and relative humidity (RH) per room. Results: Significant differences in numbers of flies and mosquitoes per room and number of insect bites per pig were observed in rooms treated with screens or screens plus insecticide compared to negative control rooms. Rooms that used screens had significantly fewer flies than rooms that used insecticide alone, although screened treatments did not differ from each other. No differences were observed across treatments in the number of fly spots per room or the level of CO2, temperature, or RH per room. Implications: Under the conditions of this study, better on-farm insect control was provided through the use of insect screens with or without insecticides. However, no conclusions can be drawn as to whether these strategies can prevent introduction of insect-borne pathogens. | ResumenObjetivo: Evaluar la eficacia de tres estrategias de control de insectos en una granja comercial porcina. Métodos: El estudio se llevó a cabo en una engorda comercial que tenía cuatro salas. Tres estrategias (tratamientos) se evaluaron: malla mosquitera en las entradas de aire de las paredes laterales, aplicación de un insecticida a base de piretroides a las paredes y pisos del edificio, la combinación de malla mosquitera e insecticida, y ningún tratamiento (control negativo). A cada sala se le asignó un tratamiento para un periodo de muestreo de dos semanas. Los parámetros específicos que se midieron cada dos semanas fueron el número de moscas y mosquitos en cada sala, número de mordeduras de insectos en cada cerdo, número de manchas de moscas por sala, y el nivel de dióxido de carbono, temperatura, y humedad relativa (RH, por sus siglas en inglés) de cada sala. Resultados: Se observaron diferencias significativas en el número de moscas y mosquitos por sala y en el número de mordeduras de insectos por cerdo en las salas que tenían malla mosquitera o malla mosquitera e insecticida comparadas con las salas control negativo. Las salas que usaron malla mosquitera tuvieron significativamente menos moscas por sala que la sala que solo utilizó insecticida, aunque los tratamientos de malla mosquitera no difirieron entre sí. No se encontraron diferencias entre los tratamientos en el número de manchas de moscas por sala, nivel de CO2, temperatura, o RH por sala. Implicaciones: Bajo las condiciones de este estudio, el mejor control de insectos se obtuvo mediante el uso de malla mosquitera con o sin insecticidas. Sin embargo, no se puede concluir si estas estrategias pueden prevenir la introducción de patógenos que porten los insectos. | ResuméObjectif: Évaluer l’efficacité de trois stratégies d’intervention de lutte contre les insectes dans un élevage de porcs commercial. Méthodes: L’étude a été menée dans un établissement de finition commercial comportant quatre salles. On a évalué l’efficacité de trois stratégies (traitements), soit la pose de moustiquaires sur les ouvertures d’aération des parois latérales du bâtiment, l’application d’un insecticide à base de pyréthroïde sur les murs et le plancher du bâtiment, et la combinaison de la pose de moustiquaires et de l’application d’insecticide. Les résultats ont été contrôlés négativement par l’absence de traitement de la quatrième salle. Les traitements ont été appliqués pendant une période d’échantillonnage de deux semaines. Les paramètres d’évaluation ont été mesurés deux fois par semaine. Ces paramètres étaient les suivants: le nombre de mouches et de moustiques par salle, le nombre de piqûres d’insecte par porc, le nombre de taches laissées par les mouches par salle ainsi que le taux de dioxyde de carbone, la température, et l’humidité relative (HR) par salle. Résultats: Il y avait une différence significative dans le nombre de mouches et de moustiques par salle ainsi que dans le nombre de piqûres d’insectes par porc dans la salle où l’on avait installé des moustiquaires et dans la salle où l’on avait installé des moustiquaires et appliqué un insecticide comparativement à la salle de contrôle négatif. Dans les salles dotées de moustiquaires, on retrouvait beaucoup moins de mouches que dans la salle où l’on avait seulement appliqué un insecticide. On n’a toutefois pas observé de différence significative entre les deux salles comportant des moustiquaires. On n’a pas non plus observé de différence significative dans le nombre de taches laissées par les mouches par salle ou dans le taux de CO2, la température, et l’HR par salle selon les types de traitements. Implications: Selon les résultats de cette étude, on peut lutter efficacement contre les insectes grâce à la pose de moustiquaires, avec ou sans application d’insecticide. Il n’a toutefois pas été possible de déterminer si ces stratégies de lutte contre les insectes permettaient d’éviter l’introduction des pathogènes transmis par ces derniers. |
Keywords: swine, swine,
insects, insect control, porcine reproductive and respiratory syndrome virus
Search the AASV web site
for pages with similar keywords.
Received: January
21, 2005
Accepted: April
29, 2005
Insects such as mosquitoes and house flies have long been known to affect the health and comfort of domestic animals, including cattle, poultry, and swine. Examples include the house fly (Musca domestica) and mosquito species such as Aedes vexans, which are frequently identified on swine farms in the midwestern United States.1,2 These insects cause discomfort to animals and farm personnel, injure skin, and transmit pathogens. Repeated feedings by mosquitoes, house flies, or both can produce skin lesions, resulting in secondary bacterial infections and substantial economic loss due to excessive trimming at slaughter. Mosquitoes possess piercing mouthparts, and female mosquitoes require a blood meal to reproduce.1 In contrast, house flies are nonbiting insects, yet possess scraping mouthparts that can access blood from skin wounds (induced by fighting or previous mosquito bites) or oronasal and ocular secretions for nutritional purposes.2 Between May and September, slaughter plants located in Minnesota have averaged $7.50 to $15.00 trim loss ($US) per carcass secondary to damage by insect bites (R. D. Moon, personal communication, 2005).
House flies and mosquitoes such as Aedes vexans have also been reported to harbor and transport economically significant swine pathogens. An example of such a pathogen that has been recovered from both mosquitoes and house flies is porcine reproductive and respiratory syndrome virus (PRRSV),3,4 the most economically significant pathogen in the swine industry today. A recently published source has estimated losses of $6.25 to $15.25 per growing pig ($US) secondary to reduced productivity and performance, elevated mortality, and the cost of additional medications and vaccinations.5 While multiple methods for controlling PRRSV have been proposed, the efficacy of these methods is not 100% across all farms. Therefore, various proposals to eliminate PRRSV from infected farms have been developed. Despite these efforts, re-infection of farms with new isolates of PRRSV is a frequent event, and the route of infection is often undetermined.6 Recently, house flies and mosquitoes have been proposed as potential routes of PRRSV transmission between farms. Under conditions that optimized transmission, house flies and mosquitoes that had fed on PRRSV-positive blood from experimentally infected pigs were capable of transmitting PRRSV to naive pigs.7,8 After ingestion by a mosquito or house fly, PRRSV resides in the insect’s intestinal tract, but does not replicate there.7-9 The ability of house flies to mechanically transport PRRSV throughout an agricultural region following contact with experimentally infected pigs has been extensively investigated, and transport of PRRSV by flies was documented 2.4 km from a facility that housed infected swine.10 Virus recovered from pooled flies was infectious by swine bioassay.10 Finally, a recent report9 indicated that flies acquired PRRSV after feeding on blood and oronasal secretions of infected pigs, and that retention of PRRSV in house flies was a function of virus load post ingestion and environmental temperature.
In order to prevent introduction of pathogens such as PRRSV to swine herds, a great deal of emphasis has been placed on developing efficacious protocols of biosecurity. Examples are use of shower in-shower out facilities for personnel, and quarantine and testing programs for incoming replacement stock.11 However, despite the potential risk of insect-borne transmission of pathogens and the cost of damaged carcasses due to insect bites, little information is currently available for control of insects on commercial swine farms. While strategies such as use of insecticides and screening of air inlets have been proposed, their ability to reduce insect populations on swine farms has not been evaluated. In contrast, these strategies have been used extensively in the poultry industry, and several population monitoring techniques have been developed to measure their efficacy, including baited jug traps and spot cards for house flies12 and carbon dioxide (CO2) light traps for mosquitoes.13 A jug trap consists of a translucent 3.8-liter plastic jug baited with commercial fly attractant. The trap is suspended from the ceiling and flies enter through openings in the sides of the jug. Spot cards are 8-cm x 13-cm white index cards that are fastened flush to the walls or rafters of a building for a period of time during which they accumulate vomitus and fecal spots from roosting flies. The number of spots per card has been used as an index of fly abundance within a building. Carbon dioxide light traps utilize emissions from dry ice and an incandescent light bulb to attract mosquitoes for capture in a netted bag-like trap.
Besides questions regarding the efficacy of screens, concerns have also been raised about whether their use could negatively affect the animal environment and potentially impair pig performance. The primary concern was whether the use of screens on the air inlets of swine facilities might reduce airflow, resulting in increased levels of CO2 as well as elevated environmental temperature and relative humidity (RH). Therefore, a study was conducted to evaluate the efficacy of screens and insecticide for control of insects and to assess their effect on the animal environment on a commercial swine farm. The study was based on the hypothesis that a combination of strategies would be more effective at controlling insects than a single strategy.
Materials and methods
Study facility
This study was conducted in a naturally ventilated commercial finishing facility in west-central Minnesota during the period of July 19 to September 13, 2004. The site was selected on the basis of the owner’s willingness to participate and his previous experience with a suspected insect-borne PRRSV infection of another of his facilities. The study barn was the only livestock facility on the site and was set in an east-west direction. The nearest swine farm was 16 km away; however, a dairy operation was located approximately 3.2 km to the southwest.
The study barn (13.3 m x 120 m) was managed all in-all out by building and consisted of four rooms separated by animal loading rooms (3 m in width; Figure 1). Each room had an entrance and an exit door, totally slatted flooring, and a capacity for 400 pigs. Animal waste drained into a 3-m deep pit divided into four equally sized compartments, one compartment per room, directly under the slats. During the study period, the rooms were ventilated by a combination of fans in the pit and curtains on the sidewall openings located on the north and south sides of the building. The facility contained no auxiliary ventilation, ie, ceiling fans. Prior to initiation of the study, the building was completely emptied of pigs and the rooms were power washed, disinfected, and allowed to dry. The entire animal airspace was then fumigated with a 0.5% short-acting pyrethrin spray (ULD-BP-50 contact insecticide formula 1; Whitmire Micro-Gen Research Laboratories, St Louis, Missouri) to reduce the resident insect population. Two days later, the barn was re-washed to remove residual insecticide prior to entry of pigs. The facility was filled over a 3-day period. Pigs entered at 25 kg and were marketed at approximately 120 kg. The pits were not emptied before or during the study. Pit additives (ie, to kill insect larvae) had never been used during the barn’s 12-year existence.
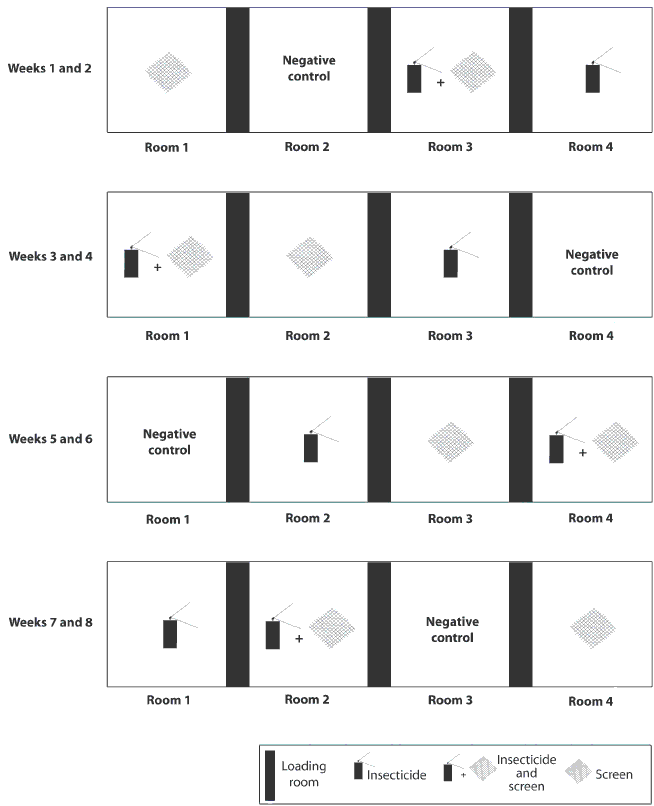
Figure 1: Diagram of facility in a study conducted to evaluate the efficacy of screens and insecticide for control of insects and to assess their effect on the animal environment on a commercial swine farm. The facility contained four rooms of equal size separated by animal loading rooms. Intervention strategies tested (treatments) included insect screens, insecticide, screens plus insecticide, and no treatment (negative control). Each treatment was randomly assigned to one of the four rooms and remained in effect for a 2-week period in each room. After each 2-week period, treatments were randomly rotated among rooms, and insecticide was washed from walls and screens were removed as required.
|
Experimental design
The study period was 8 weeks. Intervention strategies (treatments) tested included insect screens, insecticide, screens plus insecticide, and no treatment (negative control). Each treatment was randomly assigned to one of the four rooms, and remained in effect for a 2-week period in each room. After each 2-week period was completed, treatments were randomly rotated among rooms to avoid room bias (Figure 1). Between treatment periods, with pigs occupying the rooms, the walls, pen dividers, and floors of rooms treated with insecticide were power washed. Screens were power washed on a weekly basis using a low-pressure setting, and leaf blowers were used to remove dust as needed.
To assess the impact of treatment on the control of insects and the animal environment, specific insect and environmental parameters were measured, including numbers of flies and mosquitoes per room, number of fly spots per room, and number of insect bites per pig. Environmental parameters measured included level of CO2, RH, and temperature per room during each 2-week treatment period.14 Data were collected twice weekly for all parameters for a total of four measurements per 2-week treatment period, except for number of mosquitoes, which were collected once each week for a total of two measurements per treatment period.
Application of treatments
Insect screens consisted of commercially available insect screen (fiberglass mosquito netting with 1-mm square holes, 64 holes per cm2) cut to fit the sidewall openings of the room (1.6 m x 26.6 m) and stapled to the outside face of each opening. For insecticide treatments, the insecticide used was a long-acting (1 to 2 weeks) pyrethroid, (Tempo SC Ultra premise spray; Bayer, Kansas City, Missouri) that contained 11.8% beta-cyfluthrin. According to label directions, 16 mL of product was mixed with 3840 mL of water. Once per 2-week treatment period, the mixture was sprayed on vertical surfaces (walls, pen dividers, and gates) while the animals were in the room. For the purpose of the screens plus insecticide treatment, a combination of the described screen and insecticide treatments was used.
Data collection
Commercial baited jug traps (Terminator; Farnam Companies Inc, Phoenix, Arizona) were used to collect flies. The traps were baited at a concentration of 30 mL of Farnam’s terminator bait per 2 L of water per trap, and two traps were placed in each room. Traps were hung 10 cm from the ceiling, one on the north side of the room and the other on the south side, placed 6.6 m and 20 m, respectively, east of the entrance door to each room. During each collection period, jug traps were removed from the ceiling, liquid was discarded, and flies were poured into a plastic bag labeled by room number, trap number, and date. A CO2 light trap was hung in each room to collect mosquitoes, approximately 10 cm from the ceiling in the middle of the room and 13.3 m from the room’s entrance door. Upon collection, mosquitoes were handled similarly to flies. Bags containing flies and mosquitoes were then placed on ice and transported to the laboratory.
The number of fly spots was recorded using white index cards (8 cm x 13 cm) that had been taped on the east and west walls of each room, approximately 2 m from the floor and 15 cm from the entrance and exit doors. During collection periods, cards were removed from the walls and placed in plastic bags labeled as described. Laboratory personnel blinded to the relationship between room number and treatment counted the number of insects in each bag and the number of spots on each card.
To assess the number of insect bites per pig, four pigs were randomly selected in each room, and the number of bites on each pig was counted. Animals were selected on the basis of visual observation by study personnel. Different animals were used each time, and animals were not caught or herded prior to counting. An insect bite was defined as a reddened, raised, circular area on the dorsal or lateral surface of the animal.
A HOBO data logger unit (Onset Computer Corp, Pocasset, Massachusetts) was hung 5 cm from the ceiling in the middle of each room and measured temperature and RH at 30-minute intervals during the 2-week treatment period. At the end of each treatment period, data were downloaded into a computer for storage. Carbon dioxide levels were measured using a Sensidyne syringe and Gas Tech CO2 detector tubes (Elvin Safety Supply, Eden Prairie, Minnesota), which reported data in parts per million (ppm) CO2.14 For sample collection, study personnel positioned themselves in a pen approximately 2 m from the side wall on the north and south sides of each room. The syringe was held at pig level (approximately 1 m from the floor) and the sample was collected over a 1-minute time period, according to manufacturer’s recommendations. An interference level of 3000 ppm was used to determine whether gas levels in the room were within acceptable limits.14 The readings were then averaged to provide a mean CO2 level per room.
Data analysis
The effects of the four treatments on insect measurements, namely insect counts, bites, and fly spots, were tested. Environmental effects, ie, the effects of the four treatments on the level of CO2, temperature, and RH, were also tested. Treatments effects were tested using a generalized ANOVA that took into account the treatment period, timing of sampling, room, and location of the test in the room (north or south). Treatment effects were initially compared with the control using one-sided Dunnett’s multiple comparisons against the control treatment. The treatments were then compared using a Tukey HSD multiple comparison. All tests were performed using Statistix 8.0 (Analytical Software, Tallahassee, Florida).
Results
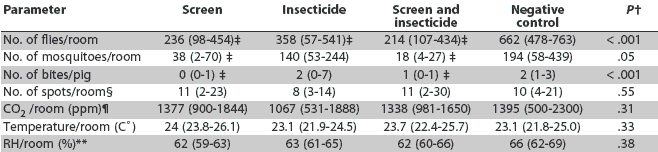
Significant treatment effects were seen in the numbers of flies and mosquitoes per room and bites per pig in comparison to the negative controls (Table 1). Numbers of flies per room were lower in all treatments than in the controls. Numbers of flies and mosquitoes were lower in treatments that used screens than in treatments using insecticide alone, although screened treatments did not differ from each other (P > .05). The number of insect bites per pig was lower by a mean of 1.5 bites per pig in rooms that used screens compared to control rooms. No significant effect (P > .05) was seen in the number of bites per pig in rooms using insecticide alone. Treatment had no significant effect on CO2 levels (P = .13), number of fly spots per card (P = .42), RH (P = .74), or temperature (P = .77), and no effect of time period was seen.
Table 1: Summary of insect and environmental parameters in a four-room commercial finishing facility in which effects of using screens, insecticide, both, or neither (negative control) were compared during an 8-week study period*
* Each room was assigned to a treatment for a 2-week period (Figure 1). Mean value and range of specific parameters across all four treatment periods are provided. Screens were fiberglass mosquito netting with 1-mm square holes, 64 holes per cm2, stapled to the outside face of the sidewall openings of the room. The insecticide was a long-acting (1 to 2 weeks) pyrethroid sprayed on vertical surfaces once per 2-week treatment period. The screen plus insecticide treatment was a combination of the described screen and insecticide treatments. The negative control group was housed in a room where neither screens nor insecticide were used. Flies were collected in baited jug traps and mosquitoes in carbon dioxide light traps. † ANOVA with parameter as dependent variable. ‡ Significantly less than negative control levels (P < .05) in one-sided Dunnett’s multiple comparisons. § Number of fly spots was recorded from white index cards taped on two walls of each room. ¶ Parts per million of carbon dioxide in room air. ** Percent relative humidity. |
Discussion
This study was based on the hypothesis that superior insect control would be demonstrated through the use of a combination of strategies versus a single strategy. The results indicated that under the conditions of the study, the use of screens was the most effective means of reducing the numbers of flies and mosquitoes per room and the number of bites per pig. Statistically significant differences in these parameters were not observed in rooms that used only screens when compared to rooms that used a combination of screens and insecticide, thereby disproving the hypothesis. Furthermore, the results also indicated that during the study period, the use of screens did not result in significant elevations in the level of CO2, temperature, or RH when these parameters were compared for nontreated rooms or rooms treated with insecticide alone. However, it must be noted that farm personnel were extremely conscientious in regard to management of the screens. While the barn was surrounded by cornfields, no trees were present on the site, and this may have limited the amount of wind-borne debris that might have impeded airflow following contact with screened inlets. Finally, historical data showed that the weather encountered during the 8-week study period was cool for the area. Specifically, the average high temperature recorded during the study was 23°C, with an average low temperature of 12°C. In contrast, in the previous year, the average high temperature during the same period was 29°C, with an average low temperature of 14°C. As a result of these cooler temperatures, there may have been fewer insects in the surrounding area, and there may have also been an indirect influence on the temperature within the barn.
Overall, the present study had several strengths. It demonstrated that when managed properly, the use of screens can significantly improve insect control on swine farms without negatively affecting the environment. The ability to randomly rotate treatments among rooms prevented any room-related bias. The fact that no significant effect was seen in the number of bites per pig in rooms that used insecticide alone, despite a significant reduction in fly numbers in rooms using insecticides, suggests that the bites were most likely caused by mosquitoes. This is a logical conclusion, since house flies are nonbiting insects, and the spectrum of activity of the insecticide used in this study did not include mosquitoes. Therefore, if insect bites are a problem on swine farms, resources should be directed toward reducing mosquito populations (ie, screens plus insecticides known to be efficacious against mosquitoes), as well as populations of biting flies. Finally, the study also tested a number of techniques previously applied in the poultry industry for monitoring insect populations. Results obtained from use of jug traps and CO2-light traps indicated that both techniques are effective for monitoring changes in insect populations following implementation of an intervention strategy on a swine farm. In contrast, significant differences in the numbers of flies were not detected through the use of spot cards. One explanation for this observation is that placement of the cards reduced the sensitivity of this method in our study. We used two cards per room, attached to opposite walls of the building, while previous work in poultry barns suggests that placement of four cards attached by a clip to the outside of the lower feed trough may improve sensitivity.15 However, it is doubtful whether cards attached this way would remain in position in swine barns. Another option is to use larger cards (eg, 13 cm x 20 cm). While the use of cards of this size has not significantly improved sensitivity in poultry barns,15 they have not been tested in swine facilities. One conclusion that could be drawn from these observations is that while jug traps are superior to spot cards for monitoring changes in fly populations, spot cards, applied according to published methods,15 might be an option if jug traps are not available.
Besides its strengths, the study had several acknowledged limitations, the most significant being site location. The study was conducted in west-central Minnesota; therefore, the results cannot be applied to farms in other areas with different climates, particularly warmer regions with a greater concentration of resident insects and extended duration of insect activity. Also, had the site been located in a heavily forested area, wind-borne debris contacting the screens (eg, leaves and twigs) might have impeded air flow. Finally, the site was located 3.5 hours from the University of Minnesota, a distance that prevented us from sampling more frequently. This limitation was especially problematic for mosquito collection, since the area had a limited supply of dry ice, an essential component of CO2-light traps.
The second limitation was the facility in which the study was conducted. The building was a specific type, was naturally ventilated, held only 1600 pigs, and was a stand-alone structure on the site. Because the facility had housed pigs for many years, a resident insect population was established internally, and prior to initiation of the study, the pit was not emptied, and pit additives to eliminate fly larva were not employed. Therefore, it was not possible to start with an insect-free facility. Furthermore, despite separation of the animal rooms by the 3-m wide loading rooms, it was not possible to prevent room-to-room movement of insects. However, these issues apparently did not impact the study, as significant differences in the measured parameters were observed between the treated rooms and the negative controls.
The third limitation was the fact that the study was conducted only one time with a duration of only 8 weeks. However, due to the short summer season in Minnesota, it was not possible to conduct the study for a longer period. In an attempt to offset this limitation, the study was conducted during a period when insect numbers historically are at their peak. Unfortunately, the summer was abnormally cool. Although it would have been ideal to repeat this study in other types of facilities, it was quite labor-intensive and it might have been difficult to find other sites in which personnel were so willing to cooperate and so dedicated to screen management.
Finally, only one insecticide, targeting flies only, was evaluated, and no attempt was made to taxonomically classify the species of insects collected or the percentage of each species in each collection. Therefore, conclusions cannot be made regarding the efficacy of the insecticide on the various species collected or the possible effects of alternative insecticide products.
Despite these limitations, it was possible to demonstrate the ability of selected intervention strategies to significantly lower insect populations without negatively impacting the animal environment. Since the swine population was PRRSV-naive and located in a region free of infected farms, no conclusions can be drawn regarding the ability of these strategies to function as efficacious biosecurity protocols against insect-borne transmission of swine pathogens. Obviously, unless the site were located in a highly dense region containing many PRRSV-infected farms, or PRRSV-contaminated insects could be released externally, this assessment would be difficult to make. Therefore, historical observations from the field involving farms that had applied these techniques or large-scale epidemiological studies of regional spread of PRRSV during periods of warm weather are needed to better understand the significance of these results.
Implications
- Under the conditions of this study, significant reductions in insect populations and insect bites in pigs may be achieved using conventional methods of control, ie, screens and insecticide.
- Under the conditions of this study, use of insect screens on the side-wall inlets is more effective than insecticide for controlling both flies and mosquitoes.
- Properly managed screens do not significantly affect temperature, RH, or CO2 concentration in the animal environment.
Acknowledgments
Funding for this study was provided by the Minnesota Pork Board and conducted in cooperation with Hilltop Swine of Morris, Minnesota.
References
1. Greenberg B. Biology of flies. In: Greenberg B, ed. Flies and Disease. Vol 2. 1st ed. New Jersey: Princeton University Press; 1973;20-95.
2. Clements AN. Trivial movement, dispersal and migration. In: Clements AN, ed. The Biology of Mosquitoes. Vol 2. 1st ed. New York, New York: CABI Publishing; 1999:319-332.
3. Otake S, Dee SA, Rossow KD, Moon RD, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica Linnaeus). Vet Rec. 2003;152:73-76.
4. Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus by mosquitoes, Aedes vexans (Meigen). Can J Vet Res. 2002;66:191-195.
5. Holck JT, Polson DD. Financial impact of PRRS. In: Zimmerman JJ, Yoon KJ, eds. National Pork Board PRRS Compendium. 2nd ed. Des Moines, Iowa; 2003:51-57.
6. Lager KM, Mengeling WL, Wesley RD. Evidence of local spread of porcine reproductive and respiratory syndrome virus. Swine Health Prod. 2002;10:167-170.
7. Otake S, Dee SA, Moon RD, Rossow KD, Pijoan C. Survival of porcine reproductive and respiratory syndrome virus in houseflies (Musca domestica Linneaus). Can J Vet Res. 2003;67:198-203.
8. Otake S, Dee SA, Moon RD, Rossow KD, Trincado C, Pijoan C. Evaluation of mosquitoes, Aedes vexans, as biological vectors of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2003;67:265-270.
9. Schurrer JA, Dee SA, Moon RD, Murtaugh MP, Finnegan CP, Deen J, Kleiboeker SB, Pijoan C. Retention of ingested porcine reproductive and respiratory syndrome virus in house flies. Am J Vet Res. 2005;66:1517-1525.
10. Schurrer JA, Dee SA, Moon RD, Rossow KD, Mahkum C, Mondaca E, Otake S, Fano E, Collins JE, Pijoan C. Spatial dispersal of porcine reproductive and respiratory syndrome virus-contaminated flies following contact with experimentally infected pigs. Am J Vet Res. 2004;9:1284-1292.
11. Moore C. Biosecurity and minimal disease herds. In: Tubbs RC, Leman AD, eds. The Veterinary Clinics of North America. Philadelphia, Pennsylvania: Harcourt Brace Javanovich; 1992:461-474.
12. Axtell RC. Integrated fly-control program for caged layer poultry houses. J Econ Entomol. 1970;63:400-405.
13. Lysyk TJ, Axtell RC. Comparison of baited jug-trap and spot card for sampling housefly populations in poultry houses. Environ Entomol. 1985;14:815-819.
14. Heber AJ. Air requirements. In: Midwest Planning Service, ed. Natural Ventilating Systems for Livestock Housing. 1st ed. Ames, Iowa: Iowa State University; 1989:2-3.
15. Beck AF, Turner EC Jr. A comparison of five house fly (Diptera: Muscidae) population monitoring techniques. J Med Entomol. 1985;22:346-348.