Original research |
Peer reviewed |
Evaluation of the efficacy of a truck-mounted tire sanitizer system during winter weather
Evaluación de la eficacia en clima de invierno de un sistema de saneamiento instalado en los neumáticos de un camión
Évaluation de l’efficacité d’un système de désinfection des pneus installé à même le camion au cours de l’hiver
Sandra F. Amass, DVM, PhD, Diplomate ABVP; Jessica L. Schneider, RVT
National Biosecurity Resource Center, Purdue University School of Veterinary Medicine, West Lafayette, Indiana. Corresponding author: Dr Sandra F. Amass, National Biosecurity Resource Center, Purdue University School of Veterinary Medicine, VCS/Lynn, 625 Harrison Street, West Lafayette, IN 47907-2026; Tel: 765-494-8052; Fax: 765-496-2608; E-mail: amasss@purdue.edu.
Cite as: Amass SF, Schneider JL. Evaluation of the efficacy of a truck-mounted tire sanitizer system during winter weather. J Swine Health Prod. 2006;14(2):101-104.
Also available as a PDF.
SummaryThe efficacy of a tire sanitizer system in reducing bacterial counts on tires in winter was tested, but could not be adequately evaluated due to minimal bacterial contamination of tires. The number of bacteria contaminating tires under different seasonal conditions was also compared. Bacterial contamination of tires varied by season. | ResumenSe probó la eficacia de un sistema de saneamiento para reducir el conteo bacteriano en los neumáticos durante el invierno, pero no se pudo evaluar adecuadamente debido a la mínima contaminación bacterina de los neumáticos. El número de bacterias que contaminan los neumáticos bajo diferentes condiciones estaciónales también se comparó. La contaminación bacteriana de los neumáticos varió según la estación. | ResuméLors de tests, il n’a pas été possible d’évaluer adéquatement l’efficacité d’un système de désinfection destiné à diminuer la numération bactérienne présente sur les pneus en hiver en raison du faible taux de contamination bactérienne relevé sur les pneus. On a aussi comparé le taux de contamination bactérienne des pneus en fonction de la saison pour conclure que cette dernière variait selon la saison. |
Keywords: swine, vehicle
tire, decontamination, biosecurity, season
Search the AASV web site
for pages with similar keywords.
Received: March
26, 2005
Accepted: June
8, 2005
We previously evaluated the efficacy of a truck-mounted tire sanitizer system after contaminating tires under natural field conditions on a typical midwestern swine farm during cool muddy fall conditions and warm dry spring conditions.1 We reported that using a tire sanitizer system with a peroxygen disinfectant reduced the number of aerobic bacteria on the footprint and tread grooves of truck tires under cool muddy fall conditions. In warm dry spring conditions, there was a reduction in the number of bacteria present on the tire footprint regardless of whether or not the tire was sprayed with disinfectant. Moreover, there was no reduction in the number of bacteria in the tread groove of the tire after spraying with disinfectant under spring conditions.
The objective of this study was to determine whether using a tire sanitizer to apply a solution of peroxygen disinfectant and antifreeze would reduce or eliminate aerobic bacteria from the tires of a veterinary truck after it was driven on roads of a pork production unit during winter conditions.
Materials and methods
Environment
Sampling for this experiment was performed during a 3-hour period on the premises of the Purdue Animal Sciences Research and Education Center Swine Unit in Montmorenci, Indiana, on February 2, 2005. Temperature and relative humidity were -7.05°C and 78.7%, respectively, at the beginning of the sampling period, and -5.17°C and 75%, respectively, at the end of the sampling period (VelociCalc Plus 8360 digital temp/RH/velocity meter; TSI Incorporated, St Paul, Minnesota). Farm roads were a mixture of gravel, grass, and soil, and main roads were asphalt. All roads were covered with snow and ice during this project.
Equipment
A 2004 three-quarter-ton pickup truck with a standard bed (Ford F250 XL; Ford Motor Company, Detroit, Michigan) was equipped with a mobile unit (Triple Crown; Porta-Vet, Hudson, Iowa ) and a tire sanitizer system (On-Board Tire Sanitizer; Monroe Snow and Ice Control, Monroe, Wisconsin). Front tires were identical tubeless radial tires (AmeriTrac LT235/85R16/M+S; General Tire, Continental Tire North America, Mount Vernon, Illinois) with four-ply treads (two polyester and two steel) and two-ply polyester sidewalls.
The mobile unit was modified to accommodate one tank supplying water to the mobile unit and a second tank supplying disinfectant solution to the tire sanitizer system. The tank connected to the tire sanitizer was filled with a solution of peroxygen compound (Virkon S; DuPont Animal Health Solutions, Wilmington, Delaware) and antifreeze (Prestone RV Antifreeze, Prestone Products Corp, Danbury, Connecticut). Two spray nozzles were mounted on each wheel well. When the sanitizer system was activated, the solution of disinfectant and antifreeze was sprayed at 45 psi onto the tread grooves, footprint (the part of the tread contacting the road), and sidewalls of the left front tire (treatment). A cut-off valve prevented disinfectant from being sprayed onto the right front tire (negative control). Rear tires were equipped in the same fashion; however, rear tires were not sampled during the experiment. When activated via an in-cab start button, the tire sanitizer system dispensed 0.47 L of disinfectant per nozzle during a 15-second interval.
Experimental design
Prior to the study, the truck was cleaned in a commercial drive-through car wash and driven to the parking lot of the swine farm. The solution of disinfectant and antifreeze was then dispensed to the left front and rear tires for a total of 30 seconds to clear dispenser lines. Ten replicates of the following protocol were then performed. Front tires were hosed with farm water until all visible disinfectant-solution residue and organic material were removed. Tires were contaminated by driving the truck on farm roads at speeds of up to 32 km per hour in a figure-eight pattern to ensure contact of both front tires with similar road substrate. Briefly, the truck was driven for 0.64 km clockwise, then 0.64 km counterclockwise, and finally, 0.16 km clockwise to the junction of the farm road and the main road. Contaminated tires were then sampled. Next, the truck was driven off farm premises directly onto the main road. Disinfectant dispensing began when all four tires contacted the main road, and continued for two consecutive 15-second intervals. The truck was driven on the main road at approximately 32 km per hour for the first 15-second interval. Braking occurred during the last 15-second interval, and the truck was stopped approximately 5 seconds before disinfectant dispensing ended. Post-treatment samples were collected immediately; thus, in the period after disinfectant dispensing ceased and before sampling, tires collected no additional material from the main road. Ten replicates enabled detection of approximately a 50% difference between samplings with 80% power.
Sampling procedure
After contamination and after treatment, a standardized area of the footprint of each front tire (approximately 11.52 cm2) was sampled using a sterile polyester-tipped applicator swab. Additionally, a 4.04 cm3 volume of the tread groove and its contents were sampled after contamination and after treatment using a sterile polyester-tipped applicator swab. Samples were processed within 4 hours of collection.
Culture methods
Culture methods have been previously published.1 Briefly, swab samples were placed in individual sterile tubes containing 2 mL of sterile chemical broth to inactivate residual disinfectant (D/E Neutralizing Broth; Becton-Dickenson, Franklin Lakes, New Jersey). This chemical broth is effective in inactivating Virkon S (Amass, unpublished data). Samples were placed on cold packs in a cooler on the farm and during transport, then refrigerated until processing. Prior to dilution and culture, all samples were mixed by hand agitation. A 100-mL aliquot of the original sample was plated directly onto 5% sheep blood agar. Additionally, serial tenfold dilutions were made using sterile D/E Neutralizing Broth, and a 100-mL aliquot of each dilution was plated directly onto 5% sheep blood agar. Samples were incubated at 36.9°C for 24 hours. Colonies of aerobic bacteria were counted and total aerobic bacteria counts were calculated. Attempts were not made to identify specific pathogens.
Preparation of disinfectant
Approximately 45.6 L of a mixture of a 2% solution of Virkon S and full-strength Prestone RV Antifreeze was prepared. Briefly, 72.8 g of Virkon S powder was added to 3.4 L of water and 1.1 L of antifreeze to prepare each 4.5 L of solution. Virkon S is approved for use on rubber surfaces, and a concentration of 2% is allowed under the general directions (Regulatory Manager, DuPont Animal Health Solutions, personal communication, 2005). Antec International (Sudbury, Suffolk, UK), original manufacturer of Virkon S, via personal communication, provided the ratio of disinfectant to antifreeze that was used in this study. The authors do not know if the addition of antifreeze might affect the efficacy of the disinfectant.
Data analysis
Graph Pad InStat version 3.00 for Windows 95 (GraphPad Software, San Diego, California) was used for statistical calculations. A Mann-Whitney test was used to compare median bacterial counts between tires before and after treatment. A Wilcoxon matched-pairs signed ranks test was used to determine whether the median of the differences between bacterial counts on the same tire before and after treatment differed significantly from zero. The standard of disinfection has been defined2 as < 1 colony forming unit (cfu) per cm2. As the limit of detection of our culture methods was approximately 3 cfu per cm2, the standard of disinfection used in this study was < 3 cfu per cm2. The number of footprints that met the standard for disinfection after treatment, or no treatment in the case of controls, was also calculated and compared between groups using the McNemar test provided by GraphPad.
Additionally, data from this experiment were compared to data from our previously published study.1 The median number of aerobic bacteria per cm2 on the footprints of tires after contamination was compared under the various environmental conditions studied using the Krusal-Wallis test (nonparametric analysis of variance) followed by Dunn’s multiple comparisons test. For all tests, P < .05 was considered statistically significant.
Results
Snow was visible on the tire footprints and was packed into the tire tread grooves after the truck had been driven on farm roads. Samples of the water used to clean the truck tires between repetitions and the disinfectant solution were sterile. The tire sanitizer functioned appropriately for the front tires, which were sampled during this study. However, the line froze and disinfectant was not applied to the left rear tire (unsampled) for the first five repetitions.
Footprint
Median bacterial counts are presented in Table 1. The median number of bacteria cultured per 11.52-cm2 area of the footprint after tires were contaminated did not differ between tires (Mann-Whitney test). Spraying the left tire for 30 seconds with disinfectant and antifreeze did not reduce the median number of bacteria cultured (Wilcoxon matched-pairs signed ranks test). The median number of bacteria cultured was higher on the untreated (right) tire after no treatment than before no treatment (Wilcoxon matched-pairs signed ranks test). After treatment or no treatment, the median number of bacteria cultured from the footprint was not different for the left tire (sprayed with disinfectant and antifreeze) compared to the right tire (untreated) (P > .05; Mann-Whitney test). Seven of ten tire footprints in the treatment group (70%) met the standard for disinfection after treatment, while six of ten tire footprints in the control group (60%) met the standard for disinfection after no treatment (P = 1.0).
Table 1: Aerobic bacterial counts on a standardized area of the footprint of a truck tire* before and after spraying† or not spraying the tire with a solution of 2% Virkon S‡ and antifreeze§ for 30 seconds
* Colony-forming units per 11.52-cm2 area. Tires were contaminated by driving the truck on unpaved, snow-covered roads and lanes of a swine farm. Only front tires were sampled for culturing. Experiment conducted February 2. † After exiting the farm road, the truck was driven on a snow- and ice-covered asphalt road. Each left tire was sprayed using two nozzles mounted in the wheel well (On-Board Tire Sanitizer; Monroe Snow and Ice Control, Monroe, Wisconsin). Tires were sprayed for 25 seconds while the truck was moving and for approximately 5 seconds after it had stopped. Right tires were not sprayed. ‡ Virkon S; DuPont Animal Health Solutions, Wilmington, Delaware. § Prestone RV Antifreeze; Prestone Products Corp, Danbury, Connecticut. ab Median bacterial counts with different superscripts were different when compared for the same tire (Wilcoxon matched-pairs signed ranks test; P = .03). |
Tread groove
Median bacterial counts are presented in Table 2. The median number of bacteria cultured per 4.04 cm3 volume of the tread groove after tires were contaminated did not differ between tires (Mann-Whitney Test). There was no difference in the median number of bacteria cultured from the left tire after it was sprayed with disinfectant and antifreeze for 30 seconds compared to the same tire before treatment (Wilcoxon matched-pairs signed ranks test). There was no difference in the median number of bacteria cultured from the tread groove of the left tire after it was sprayed with disinfectant and antifreeze compared to the untreated right tire (Mann-Whitney test).
Table 2: Aerobic bacterial counts on a standardized volume of a truck tire tread groove and contents* before and after spraying or not spraying the tire with a solution of 2% Virkon S and antifreeze for 30 seconds
* Colony-forming units per 4.04 cm3. Driving and spraying procedures described in Table 1. |
Aerobic bacteria per cm2 of tire footprint under different environmental conditions
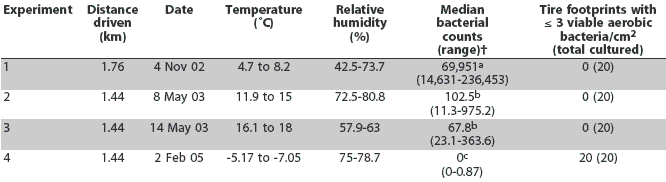
Median bacterial counts and the number of tire footprints with <= 3 viable aerobic bacteria per cm2 are presented in Table 3. The median number of aerobic bacteria cultured per cm2 of tire footprint was different among the various environmental conditions (P < .001; Kruskal Wallis test). The median number of bacteria cultured was significantly greater on November 4, 2002, than on May 8, 2003, May 14, 2003, or February 2, 2005 (P < .001; Dunn’s multiple comparison test). There was no difference between the median number of bacteria cultured on May 8, 2003 and May 14, 2003 (Dunn’s multiple comparison test). The number of bacteria cultured was significantly less on February 2, 2005, than on May 8, 2003, and May 14, 2003 (P < .001; Dunn’s multiple comparison test). During the spring and fall experiments, >= 3 viable aerobic bacteria per cm2 were cultured from all tire footprints after contamination; in contrast, during the winter experiment, < 3 viable aerobic bacteria per cm2 were cultured from all tire footprints after contamination.
Table 3: Aerobic bacterial counts on tire footprints after driving on the same roads on a single swine farm under various environmental conditions*
* Tires were contaminated by driving on the premises of the Purdue Animal Sciences Research and Education Unit in Montmorenci, Indiana, as described in Table 1 (Experiment 4) and as described for Experiments 1, 2, and 3 in Amass et al1 (2003). One truck was used for Experiments 1 through 3 and a different truck for Experiment 4. † Aerobic bacteria cultured per cm2 of tire footprint (n = 20). abc Median bacterial counts with different superscripts are different (Dunn’s multiple comparisons test; P < .001). |
Discussion
Previously, we reported that use of a tire sanitizer system with a peroxygen disinfectant reduced the number of aerobic bacteria on the footprint and tread grooves of truck tires under cool, muddy fall conditions, but the system offered little advantage during warm, dry, spring conditions.1 Similarly, the results of this study found that the system offers little advantage during winter conditions. The differences in effectiveness are likely related to the fact that bacterial numbers on tires varied with temperature and moisture conditions. We compared the raw data for the number of aerobic bacteria per cm2 cultured from the footprints of control and treated tires combined under the various environmental conditions tested. Control and treated tires had been treated identically at this point in sample collection. Bacteria were cultured from samples collected after tires had been contaminated by driving on farm premises, before any intervention occurred, in this study and in our previous studies. Aerobic bacterial counts on tire footprints were greatest in the fall and nearly absent in the winter. Moreover, in the winter trial, all footprints met the definition for the standard for disinfection of the treated surface at the end of the contamination period, before any intervention had occurred. We propose that the reason that the tire sanitizer does not appear to be effective during all seasons is that under certain environmental conditions, very few bacteria adhere to the tire surface. A statistical difference in the number of bacteria before and after disinfection is difficult to achieve when tires are minimally contaminated at the start.
In this study, we controlled for confounding factors with respect to sample collection and culture methodology by using the same experimental protocol and culture methods as in the previously published tire studies.1 However, caution must be taken in interpreting the comparisons of bacterial numbers by season, even though the same farm, driver, and roads were used. Obviously, the samples were confounded by time, as they were collected over a 28-month period. Additionally, two different trucks and sets of tires were used in these studies. Finally, the history of road use prior to our sampling was unknown, and the study included only 10 replicates.
This study and the earlier studies1 have examined only the tire sanitizer system’s effectiveness in reducing bacterial counts in tire footprints and tread grooves. Effectiveness appears to vary by season and appears to be correlated with the extent of tire contamination before intervention. Future studies should investigate whether or not viruses contaminate tires, whether the extent of viral contamination varies by season, and whether or not the tire sanitizer system has a use in ridding tires of such viruses.
Implications
- Few bacteria contaminated truck tires under the winter conditions of this study.
- Bacterial contamination of tires appears to vary with environmental conditions.
Acknowledgements
The investigators thank the American Association of Swine Veterinarians Foundation for funding this project; Monroe Liquid Systems, Leann McGowan, Tom Schartner, and Nicholas Krahenbuhl for donating and installing the tire sanitizer system and for their technical assistance during the project; Gordon Moser and Jeremiah Lehr of PortaVet for installing a dual tank mobile unit which was modified to accommodate the tire sanitizer; and Phillip Morgan for his assistance during the project.
References
1. Amass SF, Schneider JL, Ragland D, Hill MA. Pilot studies to evaluate the efficacy of a truck-mounted tire sanitizer system. J Swine Health Prod. 2003;11:277-283.
2. Tamási G. Testing disinfectants for efficacy. Revue scientifique et technique de l’Office International des Epizooties. 1995;14:75-79.