Original research |
Peer reviewed |
Genomic homology of ORF 5 gene sequence between modified live vaccine virus and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy
La homología genómica de la secuencia del gen ORF 5 entre la vacuna de virus vivo modificado y los aislamientos de reto del virus del síndrome reproductivo y respiratorio del cerdo no predice la eficacia de la vacuna
L’homologie génomique de la séquence du gène ORF 5 entre le vaccin de virus vivant modifié et les isolâtes du virus du syndrome reproducteur et respiratoire porcin ne prédit pas l’efficacité du vaccin
Tanja Opriessnig, DVM; Francisco J. Pallarés, DVM, PhD; Dachrit Nilubol, DVM, PhD; Amy L. Vincent, DVM, PhD; Eileen L. Thacker, DVM, PhD, Diplomate ACVM; Eric M. Vaughn, DVM, PhD; Michael Roof, DVM, PhD; Patrick G. Halbur, DVM, PhD
TO, FJP, PGH: Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. FJP: Departamento de Anatomía y Anatomía Patológica Comparadas, Facultad de Veterinaria, Universidad de Murcia, 30071 Murcia, Spain. DN, ALV, ELT: Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. EMV, MR: Boehringer Ingelheim Animal Health, Inc, Ames, Iowa. Corresponding author: Dr Patrick G. Halbur, Iowa State University, College of Veterinary Medicine, Veterinary Diagnostic Laboratory, Ames, IA 50011; Tel: 515-294-1950; Fax: 515-294-6961; E-mail: pghalbur@iastate.edu
Cite as: Opriessnig T, Pallarés FJ, Nilubol D, et al. Genomic homology of ORF 5 gene sequence between modified live vaccine virus and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy. J Swine Health Prod. 2005;13(5):246-253.
Also available as a PDF.
SummaryObjective: To test the efficacy of Ingelvac PRRS ATP (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri), a modified live virus (MLV) vaccine, to protect against respiratory disease and lesions induced by challenge of growing pigs with one of three porcine reproductive and respiratory syndrome virus (PRRSV) field isolates (SDSU73, VR2385, and Mn-01-A1). On the basis of genome sequencing of open reading frame (ORF) 5, these three isolates vary considerably from each other (72% to 86% homology) and from the Ingelvac PRRS ATP vaccine (76% to 89% homology). Materials and methods: Seventy-three 2-week-old segregated early weaned pigs were randomly divided into seven groups of 10 pigs and one group of three pigs. Four groups were vaccinated intramuscularly with 2.0 mL of Ingelvac PRRS ATP MLV vaccine and four groups were not vaccinated. Three vaccinated groups were inoculated intranasally 38 days later, each with a different PRRSV isolate (5.0 mL of 105.8 median tissue culture infective doses). Three nonvaccinated groups were similarly challenged. One vaccinated and one nonvac-cinated group were sham-inoculated. Necropsy was performed on all pigs 14 days postinoculation. Results: In vaccinated challenged pigs, compared to nonvaccinated challenged pigs, clinical disease was less severe (P < .05), average daily gain was greater (P < .001), and gross and microscopic lung lesions were less severe (P < .001). Implication: Under the conditions of this study, the degree of ORF 5 homology between the MLV PRRSV vaccine and the infecting strain is not a good predictor of vaccine efficacy. | ResumenObjetivo: Probar la eficacia de la Ingelvac PRRS ATP (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri), una vacuna de virus vivo modificado (MLV por sus siglas en inglés), para la protección contra la enfermedad respiratoria y las lesiones inducidas en retos a cerdos en crecimiento con uno de tres aislamientos del virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) (SDSU73, VR2385 y Mn-01-A1). Con base en la secuenciación del genoma de marco de lectura abierto (ORF por sus siglas en inglés) 5, estos tres aislamientos varían considerablemente entre ellos (homología del 72% a 86%) y de la vacuna Inglevac PRRS ATP (homología del 76% a 89%). Materiales y métodos: Setenta y tres cerdos de destete segregado temprano, de dos semanas de edad fueron divididos al azar en siete grupos de 10 cerdos y un grupo de tres cerdos. Cuatro grupos fueron vacunados intramuscularmente con 2.0 mL de la vacuna Ingelvac PRRS ATP MLV y cuatro grupos no fueron vacunados. Tres grupos vacunados fueron inoculados intranasalmente 3 días después, cada uno con un aislamiento diferente de PRRSV (dosis infecciosa media de cultivo de tejido de 5.0 ml de 105.8). Tres grupos no vacunados fueron retados de manera similar. Un grupo vacunado y otro no vacunado fueron inoculados con solución salina. Se realizó la necropsia a todos los cerdos a los 14 días post inoculación. Resultados: Al comparar los cerdos vacunados contra los no vacunados, en los vacunados la enfermedad clínica fue menos severa (P < .05), la ganancia diaria promedio fue mayor (P < .001) y las lesiones pulmonares macro y microscópicas fueron menos severas (P < . 001). Implicaciones: Bajo las condiciones de este estudio, el grado de homología del ORF 5 entre la vacuna MLV contra el PRRSV y las cepas infecciosas no es un buen predictor de la eficiencia de la vacuna. | ResuméObjectif: Tester l’efficacité de l’Ingelvac PRRS ATP (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri), un vaccin de virus vivant modifié (MLV par ses sigles en anglais), pour protéger contre la maladie respiratoire et les lésions induites par des isolâtes de défi dans des animaux d’engraissement avec un de trois virus du syndrome reproducteur et respiratoire porcin (PRRSV par ses sigles en anglais) isolâtes du champ (SDSU73, VR2385, et Mn-01-A1). Dans la base du séquençage du génome du cadre de lecture ouvert (ORF par ses sigles en anglais) 5, ces trois variez considérablement entre eux (homologie de 72% à 86%) et du vaccin l’Ingelvac PRRS ATP (homologie de 76% à 89%). Matières et méthodes: Soixante-treize porcelets de sevrage hâtive ségrége, de 2 semaines d’age ont été divisés au hasard en sept groupes de 10 porcelets et un groupe de trois porcelets. Quatre groupes ont été vaccinés de faisons intramusculaire avec 2.0 mL du vaccin Ingelvac PRRS ATP MLV et quatre groupes n’ont pas été vaccinés. Trois groupes vaccinés ont été inoculés de faisons intranasal 3 jours plus tard, chacun avec un isolément différent du PRRSV (dose médian contagieux du culture de tissu de 5.0 ml de 105.8). Trois groupes pas vaccinés ont été traits de la même façon. Un groupe vacciné et autre pas vacciné on été traite avec la solution saline. Une nécropsie a été exécutée sur tous les porcelets à 14 jours après l’inoculation. Résultats: Dans les porcelets vaccinés, comparés à cochons pas vaccinés, la maladie clinique a été moins sévère (P < .05), le gain quotidien moyen a été plus grand (P < .001), et les lésions du poumon macro et microscopiques étaient moins sévères (P < .001). Implications: Sous les conditions de cette étude, le degré de homologie du ORF 5 entre le vaccin MLV PRRSV et les souches infectant ne prédit pas l’efficacité du vaccin. |
Keywords: swine, porcine
reproductive and respiratory syndrome virus, vaccine, heterologous challenge,
efficacy
Search the AASV web site
for pages with similar keywords.
Received: February
2, 2004
Accepted: November
3, 2004
Porcine reproductive and respiratory syndrome virus (PRRSV) is the most important virus associated with porcine respiratory disease complex (PRDC) in the United States. 1,2 Considerable genetic differences among PRRSV isolates have been well documented.3-6 Differences in pathogenicity have been reported among different North American isolates7 and between North American and European isolates.8 This heterogeneity is likely to be one of the principle obstacles to effective prevention and control of the disease with commercial vaccines.9 The PRRSV genome consists of eight open reading frames (ORFs), and ORF 5 encodes the major envelope protein GP510 that is thought to be the target of neutralizing antibodies.11 The immune response against this protein likely plays an important role in protection against PRRSV. A hyper-variable region with antigenic potential has been described in ORF 5.4 This hyper-variable region is likely to have evolved because of selection of variants by host immune responses.4 It has been reported that unique amino acid substitutions in the proteins encoded by ORFs 2, 3, 4, and 5 may influence virulence, individually or in combination.12 Sequencing of ORF 5 is commonly requested by practitioners when PRRSV is isolated in field cases submitted to veterinary diagnostic laboratories. Decisions on whether to use specific modified live virus (MLV) vaccines to control PRRSV in growing pigs are sometimes based on the degree of homology between the field strain and available commercial vaccines.
Vaccines have been available for prevention and control of PRRSV since 1994. There is evidence in the literature that PRRSV vaccines have been effective under research and field conditions. Pigs vaccinated with the MLV vaccine RespPRRS (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) developed a serologic response to the vaccine and had lower respiratory disease scores and a shorter period and lower level of viremia after challenge with either a North American or a European isolate of PRRSV.13 In a field evaluation of RespPRRS, vaccinated pigs demonstrated lower clinical scores, mortality, and number of treatments, and higher average daily gain (ADG), than unvaccinated controls.14 In another study with RespPRRS, no differences in growth performance were seen between vaccinated and unvaccinated pigs.15 Prime Pac PRRS (Schering-Plough Animal Health Inc, Union, New Jersey) MLV was effective in controlling clinical PRRSV-associated respiratory disease after homologous and heterologous challenge with North American isolates in seronegative 3- to 4-week-old pigs.16 Finishing pigs from a PRRSV-infected farm in Greece were vaccinated with the European MLV PRRSV vaccine Porcilis PRRS (Intervet Inc, Millsboro, Delaware) and clinical disease was less severe, mortality was lower, and growth performance was better than in unvaccinated pigs.17
The objective of this experiment was to test the efficacy of the more recently introduced MLV PRRSV vaccine, Ingelvac PRRS ATP (Boehringer Ingelheim Vetmedica Inc), to protect against respiratory disease and lesions induced by challenge with each of three heterologous PRRSV isolates (SDSU73, VR2385, and Mn-01-A1) that vary considerably from each other and from the vaccine virus on the basis of genome sequencing of ORF 5.
Materials and methods
Study animals
Seventy-three 2-week-old segregated early weaned pigs from a herd free of PRRSV (ie, on the basis of regular serological testing with negative results) were used in this experiment. The pigs were confirmed to be seronegative when serum samples collected prior to the beginning of the study were tested by a commercial PRRSV enzyme-linked immunosorbent assay (ELISA) (HerdChek PRRS virus antibody test kit; Idexx Laboratories Inc, Westbrook, Maine).
Housing and feeding
Each treatment group was housed in a separate room with one or two raised wire deck pens, depending on group size. Each 2.9-m2 pen housed up to five pigs and contained one nipple waterer and one self feeder. Rooms and pens were identical in size, type of waterer, type of feeder, and environmental controls. The pigs were fed a complete phased diet based on corn and soybean meal. The air in the rooms was changed 15 to 20 times per minute, and temperature was maintained at approximately 22°C.
The experiment was conducted in the Iowa State University Livestock Infectious Disease Isolation Facility, and the Iowa State University Committee on Animal Care approved the experimental protocols used in this study.
Experimental design
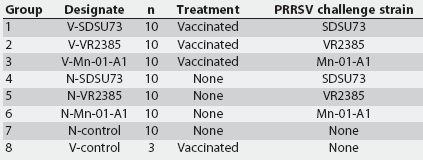
The experimental design is summarized in Table 1. At the time of delivery, pigs were randomly divided into eight groups, with each group placed in a different room. At 2 weeks of age (Day -38), pigs in Groups 1, 2, 3, and 8 were vaccinated with the MLV vaccine Ingelvac PRRS ATP. At 7.5 weeks of age (Day 0), pigs in Groups 1 through 6 were inoculated intranasally with 5.0 mL of one of three PRRSV inocula (Table 1), each with a titer of 105.8 median tissue culture infectious doses (TCID50) as previously described.8 Unchallenged groups were sham-inoculated with 5.0 mL of sterile cell culture media. Necropsy was performed on all pigs at Day 14 postinoculation, when the pigs were 9.5 weeks of age.
Table 1: Experimental design for a study in which segregated early weaned pigs were challenged with one of three field strains of porcine reproductive and respiratory syndrome virus (PRRSV), after vaccination with a modified live virus PRRS vaccine* or no vaccination
* Ingelvac PRRS ATP (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri); 2.0 mL injected intramuscularly at 2 weeks of age, 38 days prior to challenge with PRRSV. |
Vaccine and vaccination
The PRRSV strain contained in the Ingelvac PRRS ATP MLV vaccine is strain JA-142, with a restriction fragment length polymorphism (RFLP) pattern18 of 1-4-2. Pigs were vaccinated intramuscularly in the right neck with 2.0 mL of vaccine according to the manufacturer’s recommendations.
PRRS virus challenge strains
High-virulent strain SDSU73 (RFLP pattern 1-4-4) was isolated from a sow herd that had experienced a severe epidemic of "atypical" PRRS in 1996, characterized by high prevalence of abortions at all stages of gestation and higher than usual sow mortality.19 High-virulent strain VR2385 (RFLP pattern 1-3-4) was isolated from a 160-sow herd in southwestern Iowa that had experienced severe respiratory disease in 3- to 16-week-old pigs and high numbers of late-term abortions in 1991.8 Strain Mn-01-A1 (RFLP pattern 1-8-4) was recovered from a 325-sow farrow-to-finish farm20 that had experienced a severe outbreak of reproductive failure, sow mortality (10%), and high preweaning piglet mortality (55%) in 2001.
The percentages of ORF 5 nucleic acid homology between PRRSV field isolates and between each field isolate and the Ingelvac PRRS ATP vaccine strain are shown in Table 2.
Table 2: Percentages of open reading frame 5 nucleic acid homology between three field strains of porcine reproductive and respiratory syndrome (PRRS) virus and between each field strain and the Ingelvac PRRS ATP* modified live virus vaccine strain
* Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri. Restriction fragment length polymorphism pattern. NA = not applicable. |
Clinical evaluation
Rectal temperatures and clinical observations were recorded on alternate days from Day -38 until the day of inoculation (Day 0), and every day thereafter. Personnel collecting the clinical data were blinded to the virus infecting the pigs, but not to vaccination status. Clinical respiratory disease was measured as 0 = normal; 1 = sporadic sneezing or coughing; 2 = rapid respiratory rate with shallow respirations of short duration; and 3 = prolonged and labored respiration. Other clinical observations (ie, diarrhea, inappetence, and lethargy) were noted separately and were not reflected in the respiratory disease score.
The pigs were weighed at Days -39, 0, and 14 to calculate ADG before and after inoculation.
Serology
Blood was collected at Days -38, -31, -24, -17, -10, 0, 7, and 14 for virus assays and serological testing. A commercial PRRSV ELISA21 (HerdChek PRRS virus antibody test kit; Idexx Laboraotories) was used to detect and compare the anti-PRRSV antibody response of the pigs after vaccination, and prior to and after challenge. Samples with sample-to-positive (S:P) ratios >= 0.4 were considered positive.
Polymerase chain reaction
Analysis of serum for viral RNA was conducted using the nested-reverse transcriptase- (nRT-) PCR protocol for the ORF 7 region of PRRSV as previously described.22 In brief, viral RNA was extracted from 140 mL of serum using the QIAamp viral RNA kit (Qiagen, Valencia, California) in accordance with the manufacturer’s directions. For outer segment amplification, viral RNAs were amplified using reagents from the Access RT-PCR system kit (Promega, Madison, Wisconsin). Only samples negative by outer segment amplification were subjected to further testing for viral RNA by nested amplification.
Necropsy: gross and microscopic examination
Necropsy was performed on all pigs at Day 14. Pathologists were blinded to treatment group both for gross and microscopic examination. All organ systems of each pig were examined. Gross lung lesion scores were assigned to estimate the percentage of lung affected by pneumonia (0% to 100%) as previously described.8 Samples of lung, heart, kidney, ileum, spleen, tonsil, brain, liver, and tracheobronchial and mediastinal lymph nodes were collected in 10% neutral buffered formalin for histopathology. The microscopic lung sections were assigned a score for severity of interstitial pneumonia from 0 (normal) to 6 (severe diffuse interstitial pneumonia) as previously described.8 Lymphoid follicular hyperplasia, encephalitis, hepatitis, and myocarditis were scored as follows: 0 = normal; 1 = mild; 2 = moderate; and 3 = severe.
Statistical analysis
All statistical analyses in this study were performed by Statistical Analysis System (SAS) software version 9.1 (SAS Institute Inc, Cary, North Carolina). Descriptive statistics (histograms, standard deviations and means, residual plots) were performed to assess the overall quality of the data. Continuous data (weight, rectal temperature, macroscopic and microscopic lung lesions, and S:P ratios) were analyzed with a two-way-factorial analysis of variance (ANOVA) using the GLM procedure. The factors included were "vaccination status" (two levels: nonvaccinated and vaccinated) and "challenge status" (four levels: SDSU73, VR2385, Mn-01-A1, and controls). If a factorial ANOVA was significant (P < .05), pairwise testing using the Tukey’s adjustment was performed. If the interaction between "challenge status" and "vaccination status" was significant, the first step was to analyze the simple effect "challenge status" by separating the data into "vaccinated" and "nonvaccinated" categories and performing two one-way ANOVAs. In a second step, the simple effect "vaccination status" was analyzed by separating the data into challenge-status categories and then performing t-tests. Discrete data (microscopic lesions, clinical observations) for the eight groups were analyzed by the nonparametric Kruskal-Wallis one-way-ANOVA using the NPAR1WAY procedure. If the nonparametric ANOVA was significant (P < .05), Wilcoxon tests were used for pairwise testing. Response feature analysis was performed to account for repeated measurements (rectal temperature, clinical observations). First, the rectal temperature data for each pig was reduced to a pre-infection mean and a postinfection mean. A two-way-factorial ANOVA was then performed on the mean difference between the postinfection and pre-infection values. In order to simplify the analysis of clinical observations, the data were combined into groups containing three consecutive observations, and differences among these groups were compared using a nonparametric Kruskal-Wallis ANOVA. Differences between vaccinated and nonvaccinated groups were compared by t-tests.
Results
Clinical signs
An initial residual analysis of the raw rectal temperature data indicated heterogeneity of variances. This problem was corrected by log-transforming the raw data. As the interaction of challenge status and vaccination status was significant (P < .01), implying that the differences between groups in terms of challenge status were dependent on vaccination status, only simple effects were assessed. Among the vaccinated groups, transformed rectal temperature values were higher (P < .01) after PRRSV-challenge in V-Mn-01-A1 (0.16 +/- 0.04°C) than in V-VR2385 (-0.04 +/- 0.03°C) and V-Controls (-0.09 +/- 0.01°C). Transformed rectal temperature values were higher (P < .001) after PRRSV-challenge in N-SDSU73 (0.40 +/- 0.06°C) and N-Mn-01-A1 (0.47 +/- 0.05°C) than in N-VR2385 (0.15 +/- 0.06°C) and N-Controls (-0.17 +/- 0.04°C). Pairwise t-tests indicated that the mean difference between pre-inoculation and postinoculation temperatures was lower (P < .01) for all vaccinated and challenged pigs (0.08 +/- 0.03°C) than for their nonvaccinated counterparts (0.21 +/- 0.05°C).
Between Day 4 and Day 6, respiratory scores differed among groups (Kruskal-Wallis ANOVA; P < .001). Pairwise testing demonstrated that respiratory scores of vaccinated and nonvaccinated pigs inoculated with strains Mn-01-A1 (mean range 1.1 +/- 0.2) and SDSU73 (mean range 0.9 +/- 0.2) were higher (P < .01) than those of vaccinated and nonvaccinated pigs inoculated with strain VR2385 (mean range 0.3 +/- 0.1).
Average daily gain
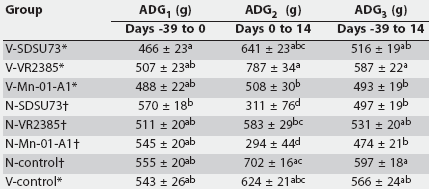
Table 3 summarizes the ADG of all groups. There was no significant interaction between vaccine and challenge isolate on ADG. In the prechallenge period (Day -39 to Day 0), there was an effect of vaccination status on weight gain (P < .01). Pairwise testing indicated that ADG was lower in V-SDSU73 than in N-SDSU73 (P < .01).
Table 3: Average daily gain (ADG) (mean +/- standard error) for the period prior to inoculation on Day 0 with porcine reproductive and respiratory syndrome (PRRS) virus (ADG1), post PRRS virus challenge (AGD2), and the entire study period (AGD3)
* Groups vaccinated with Ingelvac PRRS ATP modified live virus vaccine (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) at 2 weeks of age, 38 days before challenge with one of three field strains of PRRS virus (SDSU73, VR2385, and Mn-01-A1) or sham-inoculation (V-control). Groups not vaccinated, challenged as for vaccinated groups on Day 0 or sham-inoculated (N-control). abc Values within a column with no common superscript are different (P < .05; pairwise testing using Tukey’s adjustment). |
In the postchallenge period (Day 0 to Day 14), there was an effect of vaccination status (P < .001) and challenge status (P < .001) on weight gain. Average daily gain was lower in N-SDSU73 and N-Mn-01-A1 than in N-VR2385 and in all of the vaccinated groups (P < .001). Average daily gain was lower in N-VR2385 than in V-VR2385 (P < .001). The increase in mean ADG of vaccinated animals after challenge (Day 0 to Day 14), compared to their respective control groups, was 106%, 35%, and 73%, for the SDSU73, VR2385, and Mn-01-A1 strains, respectively.
Over the entire period of the study (Day -39 to Day 14), there was no effect of vaccination status on weight gain (P = .06); however, there was an effect of challenge status on weight gain (P < .01). Average daily gain was lower in N-Mn-01-A1 than in V-VR2385 and the N-Controls (P < .001). When vaccinated and nonvaccinated pigs were compared without taking into account the challenge strain, ADG was lower in vaccinated pigs in the prechallenge period (P < .01) (487.2 +/- 12.9 g versus 542.2 +/- 11.8 g) and higher between Days 0 and 14 (P < .001) (645.4 +/- 26.7 g versus 396.1 +/- 38.5 g), but did not differ overall (Days -39 to 14; P > .05) (531.9 +/- 13.4 versus 500.8 +/- 12.0 g).
Gross lesions
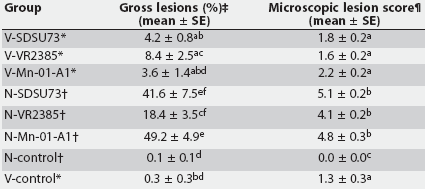
Gross lung lesions were similar in type in all PRRSV-inoculated pigs, but varied in severity among groups. The lesions primarily affected the cranial, middle, and accessory lobes and the ventromedial portion of the caudal lobes and were characterized by failure of the lung to collapse and by mottled-tan, well-demarcated areas of pneumonia. Mean percentages of lung affected by visible pneumonia are summarized in Table 4. The N-Mn-01-A1 group had the highest mean gross lung lesion score with 49.2 +/- 4.9% of the lung affected. Mean percentage of lung affected was 41.6 +/- 7.5% in N-SDSU73 and 18.4 +/- 3.5% in N-VR2385. An initial residual analysis of the raw data indicated heterogeneity of variances among treatment groups, and log-transformed data were used for further analysis. There was an effect of challenge status (P < .01) and vaccination status (P < .001) on gross lung lesions. In the nonvaccinated groups, mean lung lesion scores were higher in N-Mn-01-A1 than in N-VR2385 (P < .01). Mean gross lung lesion scores were significantly lower in the vaccinated groups compared to the respective nonvaccinated groups for all viruses (P < .001) except VR2385.
Table 4: Gross and microscopic lung lesions after challenge with porcine reproductive and respiratory syndrome virus (PRRSV) in vaccinated and nonvaccinated groups at 14 days post PRRSV inoculation
* Groups vaccinated with Ingelvac PRRS ATP modified live virus vaccine (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) at 2 weeks of age, 38 days before challenge with one of three field strains of PRRS virus (SDSU73, VR2385, and Mn-01-A1) or sham-inoculation (V-control). Groups not vaccinated, challenged at the same time as vaccinated groups or sham- inoculated (N-control). Percentage of lung affected by visible pneumonia. ¶ Interstitial pneumonia score (range 0 to 6) by microscopic evaluation. abcdef Values within a column with no common superscript are different (P < .05; pairwise testing using Tukey’s adjustment). |
Microscopic lesions
Microscopic lung lesions were similar in all PRRSV-inoculated pigs, but differed in severity among groups. Lesions were characterized by septal infiltration with mononuclear cells, type 2 pneumocyte hypertrophy and hyperplasia, and alveolar exudate consisting of mixed inflammatory cells and necrotic debris. Microscopic lung lesions scores are summarized in Table 4. Log transformation was used to account for heterogeneity in variances. There was an effect of vaccination status (P < .001) on microscopic lung lesions. Scores were lower in the vaccinated groups for all viruses. The mean score in the vaccinated, unchallenged control group indicated mild multifocal interstitial pneumonia in these pigs. No microscopic lung lesions were detected in the N-Control group.
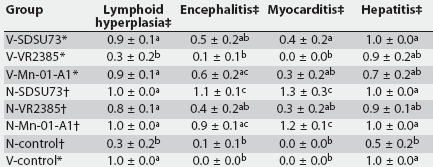
Microscopic lesions in lymphoid tissues were characterized by mild to moderate lymphoid follicular hyperplasia in the PRRSV-infected groups. Microscopic lesions in the brain were characterized by lympho-plasmacytic infiltration with perivascular cuffing and gliosis. There was also mild multifocal lymphoplasmacytic myocarditis and hepatitis in the PRRSV-infected groups. The mean scores for severity of lymphoid hyperplasia, lymphoplasmacytic encephalitis, myocarditis, and hepatitis are summarized in Table 5. There were differences between groups for lymphoid hyperplasia, encephalitis, myocarditis, and hepatitis. Lymphoplasmacytic encephalitis was more severe in the N-SDSU73 pigs than in all other groups except N-Mn-01-A1 and V-Mn-01-A1. The mean scores for lymphoplasmacytic myocarditis in N-SDSU73 and in N-Mn-01-A1 were higher (P < .001) than the mean scores in N-VR2385 and all of the vaccinated groups. When all vaccinated, challenged groups were considered together and compared with nonvaccinated, challenged groups, lymphoid hyperplasia (P = .02), lympho-plasmacytic encephalitis (P < .01), and myocarditis (P < .001) were less severe in vaccinated groups, but lymphoplasmacytic hepatitis did not differ.
Table 5: Mean microscopic lesion scores for lymphoid follicular hyperplasia, encephalitis, myocarditis, and hepatitis at 14 days postinoculation with porcine reproductive and respiratory syndrome virus (PRRSV)
* Groups vaccinated with Ingelvac PRRS ATP modified live virus vaccine (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) at 2 weeks of age, 38 days before challenge with one of three field strains of PRRS virus (SDSU73, VR2385, and Mn-01-A1) or sham-inoculation (V-control). Groups not vaccinated, challenged at the same time as vaccinated groups or sham-inoculated (N-control). Scored as 0 = normal; 1 = mild; 2 = moderate; 3 = severe. abc Values within a column with no common superscript are different (P < .05; pairwise testing using Tukey’s adjustment). |
Antibody response
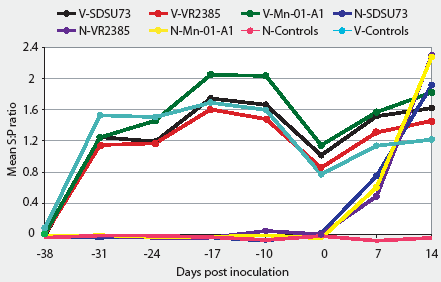
The serological profiles of all vaccinated groups were similar over the duration of the experiment (Figure 1). Vaccinated groups seroconverted between Day -38 and Day -31 (the week after vaccination). The S:P ratios were highest at Day -17, and decreased until the day of inoculation, when they started to rise again. The nonvaccinated groups remained seronegative until the day of inoculation and seroconverted by Day 7, reaching their highest S:P ratio at the termination of the experiment (Day 14). Unchallenged control pigs remained seronegative throughout the study.
Figure 1: Groups of 10 pigs vaccinated at 2 weeks of age with a modified live virus porcine reproductive and respiratory syndrome (PRRS) vaccine (Ingelvac PRRS ATP; Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) were inoculated intranasally 38 days later (Day 0) with one of three field strains of PRRS virus (Groups V-SDSU73, V-VR2385, and V-Mn-01-A1). Nonvaccinated groups of 10 pigs were inoculated with the same dose of the same strains (Groups N-SDSU73, N-VR2385, and N-Mn-01-A1). One group (10 pigs) was vaccinated but not challenged (V-control) and one group (three pigs) was neither vaccinated nor challenged (N-control). An enzyme-linked immunosorbent assay (HerdChek PRRS virus antibody test kit; Idexx Laboratories Inc, Westbrook, Maine) was used to detect and compare antibody responses after vaccination, and prior to and after challenge. Samples with sample-to-positive (S:P) ratios >= 0.4 were considered positive. Mean S:P ratios were compared using a two-way factorial ANOVA followed by pairwise testing using Tukey’s adjustment. Mean S:P ratios were higher (P < .05) in vaccinated compared to nonvaccinated groups at Days -31, -24, -17, -10, and 0. Mean S:P ratios were lower in N-VR2385 than in the other vaccinated challenged groups (P < .05), and in N-SDSU73 and N-Mn-01-A1 compared to V-SDSU73 and V-Mn-01-A1 (P < .05) at Day 7. There were no differences among challenged groups at Day 14 (P > .05)
|
Polymerase chain reaction
All animals in the nonvaccinated groups were positive for PRRSV nucleic acids in serum by nRT-PCR at Days 7 and 14. By Day 7, 90% of pigs in V-SDSU73, 80% of pigs in V-VR2385, and 100% of pigs in V-Mn-01-A1 were PCR-positive The percentage of PCR-positive animals decreased by Day 14 to 70% of pigs in V-SDSU73 and 50% of pigs in V-VR2385. In the V-Control group, 100% of pigs were PCR-positive at Day 7 and 33% at Day 14. The N-Control group remained PCR-negative throughout the study (data not shown).
Discussion
The three PRRSV strains used in this experiment were isolated from three unrelated herds, each with a history of severe reproductive and respiratory disease, and were collected over a 10-year period. All are considered high-virulent US isolates compared to other strains used in the same growing pig model.7,8,23 Strains Mn-01-A1 (from a 2001 outbreak) and SDSU73 (from a 1996 outbreak) were as virulent as or more virulent than strain VR2385 (from a 1991 outbreak), which we have used for several years as the standard for high virulence in our PRRSV respiratory disease model. In pigs inoculated with strains Mn-01-A1 and SDSU73, ADG was lower postchallenge and gross lung lesions and myocarditis were more severe than in pigs inoculated with strain VR2385. These three isolates differ from each other and from the vaccine used in this experiment in predicted RFLP patterns and in genomic sequence of the ORF 5.
In this study, the Ingelvac PRRS ATP vaccine was efficacious. Average daily gain was higher and gross and microscopic lesions were less severe in vaccinated pigs challenged with each of the three PRRSV isolates. The Ingelvac PRRS ATP strain (JA-142) was also used in a recent experiment as part of an attenuated, multi-strain PRRS vaccine.24 This vaccine provided a high level of protective immunity. Fevers were lower and macroscopic lung lesions were less severe following heterologous challenge with PRRSV strain SDSU73. The protective effect of the multi-strain vaccine was in part attributed to the inclusion of the Ingelvac PRRS ATP and Ingelvac PRRS MLV strains. A similar vaccine not containing those strains showed little or no protective effect.25
In this study, mean ADG in the prechallenge period (Days -39 to 0) was lower for vaccinated pigs, suggesting that the MLV vaccine may have an initial negative effect on growth. However, in the postchallenge period (Days 0 to 14), mean ADG increased 35% to 106% in vaccinated pigs compared to their nonvaccinated counterparts. In field trials with the RespPRRS14 and Porcilis PRRS17 vaccines, the vaccine-associated increase in ADG was only 15% and 7%, respectively. The presence of pathogens other than PRRSV, less optimal environmental factors, and other variables may have negatively influenced ADG in those field trials.
It is important to point out that the Ingelvac PRRS ATP vaccine was used 38 days prior to challenge in this study. Some vaccine failures reported under field conditions may be associated with exposure to PRRSV soon after weaning, prior to development of vaccine-induced protective immunity. Mengeling et al24 have demonstrated that attenuated vaccine virus may still be present in the serum at high titers 3 weeks after vaccination, and the degree of protective immunity against heterologous challenge is likely to parallel the clearance of vaccine virus. On the basis of our current work, we conclude that PRRSV MLV vaccines such as Ingelvac PRRS ATP can provide heterologous immunity in growing pigs if used 5 weeks prior to expected exposure. Evidence of vaccine-induced clinical disease and gross lesions was lacking.
The Ingelvac PRRS ATP vaccine did not provide full protection against disease or PRRSV-associated lesions. Vaccinated pigs had substantially and significantly less severe clinical signs associated with PRRSV; however, observers responsible for clinical evaluation and scoring were not blinded to vaccination status, which might have caused some bias. Gross and microscopic lesions associated with PRRSV were recorded in a blinded fashion and were less severe in vaccinated pigs. In addition, mean ADG was higher for vaccinated pigs. Viremia following challenge was shorter in vaccinated groups inoculated with strains SDSU73 and VR2385. In the study by Gorcyca et al13 with a similar PRRSV MLV vaccine (RespPRRS), a maximum of 30% of vaccinated pigs tested by virus isolation were viremic on days 1, 3, 5, 7, and 9 post challenge, with no detectable virus in serum by day 11 post challenge. The difference between experiments in the period of viremia detected in vaccinated pigs after challenge may be due to use of a more sensitive assay (nRT-PCR) in our study compared to virus isolation used in the other study, or to the high virulence of the viruses used to challenge the pigs in the current experiment.
The GP5 protein is thought to play an important role in production of neutralizing antibodies,11 and sequencing of ORF 5 is commonly requested from veterinary diagnostic laboratories by practitioners following isolation of PRRSV. Meng et al4 have demonstrated that genetic sequencing of isolates cannot determine virulence. It has also been reported that unique amino acid substitutions in the ORF 5 may have an effect on virulence.12 In this current experiment, the most virulent strains, defined by clinical disease and gross lesions, were SDSU73 and Mn-01-A1.
Decisions on whether to use MLV vaccines to control PRRSV in growing pigs are sometimes based on the degree of ORF 5 homology between a field strain and available commercial vaccines. Labarque et al26 demonstrated that protection against PRRSV-induced pneumonia was better after vaccination with an antigenically and genetically more related strain. However, in this current experiment, the greatest reduction in lung lesions was found in the vaccinated animals inoculated with strains SDSU73 and Mn-01-A1, which have the lower percentages of ORF 5 homology with the vaccine strain.
Although the PRRSV isolates used in this study are considered to be genetically quite different from the vaccine strain, the vaccine was highly effective in reducing severity of PRRSV-associated disease and lesions. Therefore, the degree of genetic homology of the ORF 5 between the MLV PRRS vaccine used in this study and the challenge isolate was not a good predictor of vaccine efficacy. Genetic sequencing remains a useful epidemiological tool for determining whether a field isolate may be a derivative of an MLV vaccine23 and for tracking the spread or introduction of PRRSV isolates within production systems or geographical regions, but should not be used to select PRRSV vaccines or predict vaccine efficacy.
Implications
- Under the study conditions, ADG was higher and gross and microscopic lesions were less severe when pigs were vaccinated with an MLV PRRS vaccine prior to challenge with a heterologous PRRSV.
- Under the conditions of this study, vaccination with an MLV PRRS vaccine did not protect against viremia in pigs challenged with virulent PRRSV.
- The degree of genetic homology between MLV PRRSV vaccine and the infecting strain is not a good predictor of vaccine efficacy.
Acknowledgements
This work was funded in part through a grant from Boehringer Ingelheim Vetmedica, Inc. The authors recognize the contribution of the PRRSV Mn-01-A1 isolate from Drs Kurt Rossow and Marty Mohr.
References
1. Harms PA, Halbur PG, Sorden SD. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J Swine Health Prod. 2002;10:27-30.
*2. Halbur PG. Porcine viral respiratory diseases. 15th IPVS Cong. Birmingham, England. July 1998:1-10.
3. Meng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the USA and Europe. Arch Virol. 1995;140:745-755.
4. Meng XJ, Paul PS, Halbur PG, Morozov I. Sequence comparison of open reading frames 2 to 5 of low virulence and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1995;76:3181-3188.
5. Meng XJ, Paul PS, Morozov I, Halbur PG. A nested set of six or seven subgenomic mRNAs is formed in cells infected with different isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1996;77:1265-1270.
6. Allende R, Lewis TL, Lu Z, Rock DL, Kutish GF, Ali A, Doster AR, Osorio FA. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural proteins coding regions. J Gen Virol. 1999;80:307-315.
7. Halbur PG, Paul PS, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparative pathogenicity of nine US porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a five-week-old cesarean-derived, colostrum-deprived pig model. J Vet Diagn Invest. 1996;8:11-20.
8. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;34:648-660.
9. Meng XJ. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol. 2000;74:309-329.
10. Meulenberg JJ, Petersen-Den Besten A, De Kluyver EP, Moormann RJ, Schaaper WMM, Wensvoort G. Characterization of proteins encoded by ORF2-7 of Lelystad virus. Virology. 1995;206:155-163.
11. Pirzadeh B, Dea S. Monoclonal antibodies to the ORF5 product of porcine reproductive and respiratory syndrome virus define linear neutralizing determinants. J Gen Virol. 1997;78:1867-1873.
12. Yang SX, Kwang J, Laegreid W. Comparative sequence analysis of open reading frames 2 to 7 of the modified live vaccine virus and other North American isolates of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1998;143:601-612.
*13. Gorcyca D, Schlesinger K, Chladek D, Behan W, Polson D, Roof M, Doitchenoff D. RespPRRS: a new tool for prevention and control of PRRS in pigs. Proc AASP. Omaha, Nebraska. 1995:1-22.
*14. Gorcyca D, Spronk G, Morrison R, Polson D. Field evaluation of a new MLV PRRS virus vaccine: Applications for PRRS prevention and control in swine herds. Proc AASP. Omaha, Nebraska. 1995:401-411.
15. Sornsen SA, Zimmerman JJ, Polson DD, Roof MB. Effect of PRRS vaccination on average daily gain: a comparison of intranasal and intranasal-intramuscular administration. Swine Health Prod. 1998;6:13-19.
*16. Hesse RA, Couture LP, Lau ML, Wasmoen TL, Doster AR, Cooper AR. Efficacy of Prime Pac PRRS in controlling PRRS respiratory disease: homologous and heterologous challenge. Proc AASP. Quebec, Canada. 1997:137-144.
17. Mavromatis I, Kritas SK, Alexopoulos C, Tsinas A, Kyriakis SC. Field evaluation of a live vaccine against porcine reproductive and respiratory syndrome in fattening pigs. J Vet Med B. 1999;46:603-612.
18. Wesley RD, Mengeling WL, Lager KL, Clouser D, Landgraf JG, Frey ML. Differentiation of a porcine reproductive and respiratory syndrome virus vaccine strain from North American field strains by restriction fragment length polymorphism analysis of ORF5. J Vet Diagn Invest. 1998;10:140-144.
19. Mengeling WL, Lager KM, Vorwald AC. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of "atypical" PRRS. Am J Vet Res. 1998;59:1540-1544.
*20. Mohr MF, Rossow KD. Unique porcine reproductive and respiratory syndrome virus (PRRSV) sequences identified in four Minnesota swine herds. Proc Am Assoc Vet Lab Diagnost. St. Louis, Missouri. 2002:42.
21. O´Connor M, Fallon M, O´Reilly PJ. Detection of antibody to porcine reproductive and respiratory syndrome (PRRS) virus: reduction of cut-off value of an ELISA, with confirmation by immunoperoxidase monolayer assay. Irish Vet J. 2002;55:73-75.
22. Christopher-Hennings J, Nelson EA, Nelson JK, Hines RJ, Swenson SL, Hill HT, Zimmerman JJ, Katz JB, Yaeger MJ, Chase CCL, Benfield DA. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J Clin Microbiol. 1995;33:1730-1734.
23. Opriessnig T, Halbur PG, Yoon KJ, Pogranichniy RM, Harmon KM, Evans R, Key KF, Pallares FJ, Thomas P, Meng XJ. Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory virus (PRRSV) vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J Virol. 2002;76:11837-11844.
24. Mengeling WL, Lager KM, Vorwald AC, Clouser DF. Comparative safety and efficacy of attenuated single-strain and multi-strain vaccines for porcine reproductive and respiratory syndrome. Vet Microbiol. 2003;93:25-38.
25. Mengeling WL, Lager KM, Vorwald AC, Koehler KJ. Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2003;93:13-24.
26. Labarque G, Van Gucht S, Van Reeth K, Nauwynck H, Pensaert M. Respiratory tract protection upon challenge of pigs vaccinated with attenuated porcine reproductive and respiratory syndrome virus vaccines. Vet Microbiol. 2003;95:187-197.
* Non-refereed references.