Original research |
Peer reviewed |
Environmental temperature, space allowance, and regrouping: Additive effects of multiple concurrent stressors in growing pigs
Temperatura medio ambiental, espacio por animal y reagrupación: Efecto aditivo de múltiples factores simultáneos de estrés en cerdos de crecimiento
La température environnemental, désignation d’espace, et réorganisation: Effets additifs des multiple facteurs concourant qui causent de stress dans les animaux en engraissement
Y. Hyun, PhD; M. Ellis, PhD; S. E. Curtis, PhD; R. W. Johnson, PhD
Department of Animal Sciences, University of Illinois, 1207 W Gregory Dr, Urbana, Illinois; Corresponding author: Dr Michael Ellis, Department of Animal Sciences, University of Illinois, 216 Animal Sciences Laboratory, 1207 W Gregory Dr, Urbana, IL 61801; Tel: 217-333-6455; Fax: 217-333-7861; E-mail: mellis7@uiuc.edu
Cite as: Hyun Y, Ellis M, Curtis SE, et al. Environmental temperature, space allowance, and regrouping: Additive effects of multiple concurrent stressors in growing pigs. J Swine Health Prod. 2005;13(3):131-138.
Also available as a PDF.
SummaryObjective: To determine the effects of multiple concurrent stressors on hematologic and behavioral measurements in growing pigs. Materials and methods: Two hundred and fifty-six growing pigs (Yorkshire x Hampshire or purebred Duroc barrows and gilts, initial body weight 34.7 +/- 0.5 kg) were used in a randomized block design with a 2 x 2 x 2 factorial arrangement of treatments to determine effects on hematologic and behavioral measures of environmental temperature (constant-thermoneutral at 24°C or cycling-high from 28°C to 34°C), space allowance (0.56 or 0.25 m2 per pig), and regrouping (static, ie, not regrouped, or regrouped at start of Weeks 1 and 3 of the study). After a 7-day adjustment period at 24°C and 0.56 m2 of space per pig, treatments were imposed for the study period of 4 weeks. Results: At end of Week 4, the cycling-high temperature treatment group had lower lymphocyte concentrations, higher neutrophil concentrations, and, consequently, higher neutrophil:lymphocyte (N:L) ratios (P < .05). Neither space allowance nor regrouping influenced N:L ratio. No stressor affected hematocrit (HCT) or plasma cortisol concentration. However, cortisol and N:L ratio were higher and HCT was lower as number of stressors increased. Pigs on the cycling-high temperature treatment were less active than those on the constant-thermoneutral treatment. Standing activity was higher and lying activity was lower as number of stressors increased (P < .05). Implications: The three stressors tested in this study influenced hematological and behavioral measurements indicative of stress in growing pigs, and some of these effects were additive. | ResumenObjetivo: Determinar los efectos de múltiples factores simultáneos de estrés sobre las medidas hematológicas y de conducta en cerdos de crecimiento. Materiales y Métodos: Doscientos cincuenta y seis cerdos en crecimiento (Yorkshire x Hampshire o de raza pura Duroc, machos castrados o hembras , con un peso inicial de 34.7 +/- 0.5 kg) se utilizaron en un diseño de bloque al azar, con un modelo factorial de 2 x 2 x 2 para determinar los efectos de la temperatura ambiental (constante termoneutral a 240C ó en ciclo alto de 280C a 340C), espacio (0.56 ó 0.25 m2 por cerdo), y reagrupación (estática o reagrupación en las Semanas 1 y 3) sobre las medidas hematológicas y.de conducta. Después de un periodo de adaptación de 7 días a 240C y 0.56 m2 por cerdo, se asignaron los diferentes tratamientos para el periodo de estudio de 4 semanas. Resultados: Al final de la semana 4, el grupo con temperatura de ciclo alto tuvo un porcentaje más bajo de linfocitos, un porcentaje más alto de neutrófilos y consecuentemente una proporción más alta (P < .05) de neutrófilos:linfocitos (N:L). Ni la asignación de espacio, ni la reagrupación influenciaron la proporción de N:L. Ningún factor de estrés afectó la concentración de cortisol en plasma ó el hematocrito (HCT por sus siglas en inglés). Sin embargo, la proporción de N:L y de cortisol fue más alta y el HCT fue más bajo al aumentar el número de factores de estrés. Los cerdos en el tratamiento de temperatura de ciclo alto presentaron menos actividad que aquellos en el tratamiento termoneutral constante. La actividad de los cerdos en pie fue más alta y la de cerdos en reposo fue más baja al aumentar el número de factores de estrés. (P < .05) Implicaciones: Los tres efectos de estrés asignados en este estudio influenciaron las medidas sanguíneas y de conducta que son indicativas de estrés en cerdos de crecimiento, además se observó cierto efecto aditivo. | ResuméObjectif: Déterminer les effets des multiples facteurs concourant de stress, sur les mesures hématologiques et de la conduite dans les animaux en engraissement. Matières et méthodes: Deux cents cinquante six cochons de engraissement (Yorkshire x Hampshire ou Duroc de race pure, male castre o cochettes, avec un poids initial de 34.7 +/- 0.5 kg) ont été utilisés dans un dessin du bloc au hasard, avec un arrangement factoriel de 2 x 2 x 2 pour déterminer des effets de la température environnemental (termoneutral constant à 240C ou de cycle haut de 280C à 340C), espace (0.56 ou 0.25 m2 par animal), et réorganisation (statique ou a réorganisation aux semaines 1 et 3) sur les mesures hématologiques et de la conduite. Après une période d’ajustement de 7 jours à 240C et 0.56 m2 d’espace par animal, les traitements ont été désignés pour la période d’étude de 4 semaines. Résultats: À la fin de la semaine 4, le groupe de la température de cycle haut a eu un pourcentage inférieur de lymphocytes, un pourcentage plus haut de neutrophils, et, par conséquent, un ratio plus haut (P < 05) de neutrophil:lymphocyte (N:L). Ni la désignation d’espace, ni la réorganisation n’ont influencé le ratio N:L. Aucun facteur de stress n’a affecté l’hematocrit (HCT par ses initiales en anglais) ou la concentration du cortisol de plasma. Cependant, le ratio du cortisol et du N:L a été plus haut et le HCT a été inférieur comme le nombre de facteurs qui causent stress a augmenté. Les animaux dans le traitement de la température de cycle haut ont été moins actifs que ceux sur le traitement thermoneutral constant. L’activité debout a été la plus haute et l’activité menteuse a été inférieure comme le nombre des facteurs de stress a augmenté (P < 05). Implications: Les trois facteurs que causent stress désigné dans cette étude ont influencé les mesures du sang et de la conduite qui sont indicatifs de stress dans les animaux en engraissement, avec quelques effets additifs. |
Keywords: swine, temperature,
space, regrouping, stress
Search the AASV web site
for pages with similar keywords.
Received: August
25, 2003
Accepted: June
30, 2004
High environmental temperatures,1,2 inadequate floor-space allowance,3-5 and social disruptions due to regrouping6,7 cause stress and decrease growth rate in pigs. In commercial operations, pigs often experience more than one stressor simultaneously. Understanding effects of multiple concurrent stressors is just as important as understanding respective effects of any single stressor. Multiple concurrent stressors in chicks (high aerial ammonia, beak-trimming, coccidiosis, intermittent electric shock, heat stress, and noise) had linearly additive effects on plasma cortisol concentration and blood heterophil:lymphocyte (H:L) ratio.8 Adrenal hypertrophy, which enhances the adrenocortical response to ACTH, has been found in chronically stressed pigs.9
There has been limited research investigating the impact of multiple concurrent stressors in pigs. Hyun et al,10 in the part of the study reported here that evaluated growth performance, subjected pigs to diurnal temperature cycling from 28°C to 34°C, restricted floor-space allowance, and regrouping. Experienced singly, each of these stressors resulted in a lower body weight (BW) gain, and, as in chicks,11 effects of the three stressors experienced concurrently were additive. Plasma cortisol concentration and neutrophil:lymphocyte ratio (N:L ratio) have been reported to be higher in pigs stressed by shipping.12 Kelley13,14 reported that heat stress affects feed intake, BW gain, certain blood measurements, and the cellular immune system. Heat and social stressors interacted in their effects on the pig’s immune system.15
Influences of multiple concurrent stressors on hematologic and behavioral characteristics in pigs have not been reported. The objective of the current study was to determine the effects of three common stressors, namely, environmental temperature, reduced space allowance, and regrouping, on hematologic and behavioral characteristics in growing pigs, using the animals from the study of Hyun et al10 that had previously reported on growth performance.
Materials and methods
Experimental design and facilities
Experimental design was a randomized block with a 2 x 2 x 2 factorial arrangement of treatments: environmental temperature regimen (constant-thermoneutral or cycling-high); floor-space allowance (0.25 m2 or 0.56 m2 per pig); and regrouping (static or mixed groups). For the constant-thermoneutral treatment, the temperature in the room was held at 24°C; for the cycling-high treatment, the temperature was programmed to cycle from a low of 28°C between midnight and 6:00 am to a high of 34°C between 7:00 am and 11:00 pm. The experiment comprised two trials, with two replicates per trial, and used two rooms, each with eight pens. Each pen had a two-place feeder, a nipple waterer, and a partially slotted concrete floor. Pen was the experimental unit. Pigs had free access to feed and water.
The experimental diets were based on corn-soybean meal and formulated to meet or exceed NRC (1988)16 nutrient requirements for grower pigs (17% crude protein, 0.9% lysine, and 3296 Kcal metabolizable energy per kg).
The two rooms were identical in design (13.1 m x 11.7 m) and were mechanically ventilated, with adjustable inlets along one side providing adequate air distribution. Air-exchange rate was adjusted so as to be similar in both rooms to minimize differences in air quality. Aerial ammonia and hydrogen sulfide concentrations were measured using colorimetric indicator tubes (Mine Safety Appliances Co, Pittsburgh, Pennsylvania) in both rooms once in each of Weeks 1 and 3 of the study period.
Animals
The protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Illinois. Yorkshire x Hampshire or purebred Duroc barrows and gilts (n = 256; initial BW 34.7 +/- 0.5 kg) were assigned to outcome groups of eight on the basis of litter, sex, and BW, and then randomly allocated to one of eight treatments to form experimental groups of eight pigs per pen with equal numbers of each genotype and sex in each of the 32 pens (eight pens x two rooms x two trials) used in the two trials.
Room assignments and regrouping strategy
For Trial One, rooms were randomly allocated to temperature treatment. In each room, the eight groups were randomly allocated to space-allowance and regrouping treatments. For Trial Two, the temperature treatments were switched between rooms. Pigs were allowed a 1-week acclimatization period at 0.56 m2 per pig and constant-thermoneutral temperature (24°C). Immediately prior to the start of each trial, temperature treatments were imposed and pen size adjusted to give two space allowances.
Regrouping was applied twice during each trial: on Day 1 of Week l and on Day 1 of Week 3. Within each room, there were two pens on each space x regrouping treatment. On Day 1 of Week 1, four pigs from a pen on the regrouping treatment were switched with four from a pen on the same treatment in the same room. Then, on Day 1 of Week 3, this procedure was repeated, with pigs not moved earlier being switched with those on the same treatment in the same room.
Immunosuppression and neuroendocrine measurements
At the end of Week 4, a 5-mL blood sample was collected by venapuncture from each of four pigs randomly selected from each pen. Blood smears were immediately made and stained in duplicate (Camco Quik Stain II; Baxter Healthcare Corp, McGaw Park, Illinois) for counting neutrophils and lymphocytes.17 The rest of the sample was split between two microhematocrit tubes for hematocrit (HCT) determination.
The other four pigs in each pen were subjected to a functional test of the hypothalamic-pituitary-adrenal axis.18 At approximately 7:00 am on sampling day, pigs were injected with dexamethasone (Steris Laboratories, Phoenix, Arizona; 0.2 mg per kg BW) to suppress endogenous ACTH production. Three hours later, a blood sample was collected from the vena cava into a tube containing heparin as anticoagulant. Samples were collected within 2 minutes of the start of restraint to avoid hematological changes induced by stress. Pigs then received an intramuscular injection of ACTH (Sigma Chemical, St Louis, Missouri; 2 IU per kg BW),19 and blood samples were collected 90 minutes later. Plasma was harvested and stored at -20°C. Plasma cortisol concentration was measured using a radioimmunoassay kit (ICN Biomedicals Inc, Costa Mesa, California).19,20 Stimulated cortisol levels were determined by subtracting baseline cortisol levels after dexamethasone injection from peak cortisol levels following ACTH injection.
Behavior measurements
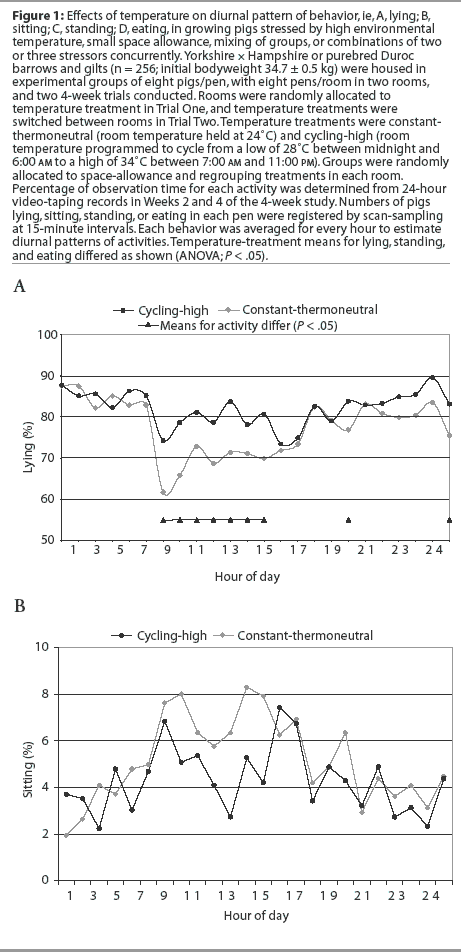
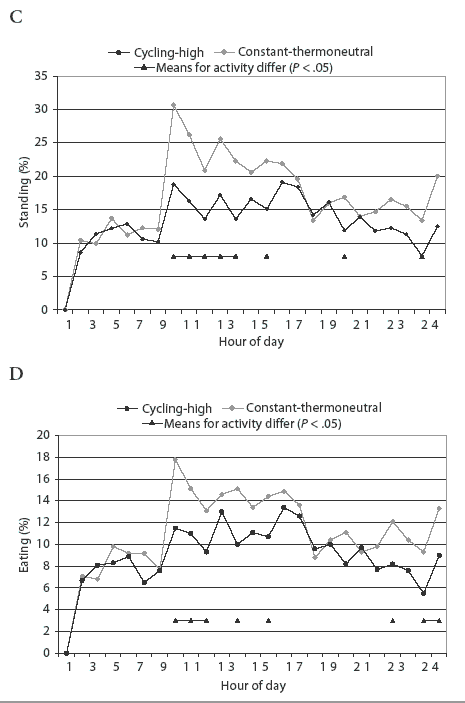
Activity of pigs in each pen was video-recorded for 24 hours in Week 2 and again in Week 4 of each trial. Numbers of pigs lying, sitting, standing, or eating in each pen were registered from video-records by scan-sampling at 15-minute intervals. Each behavior was averaged for every hour to estimate diurnal patterns of activities.
Statistical analysis
All data were tested for normality using the Proc Univariate procedure of SAS Version 8 (SAS Institute Inc, Cary, North Carolina). All variables were normally distributed. Analysis of variance was carried out using the Proc Mixed procedure of SAS to determine effects of treatments on cortisol, HCT, neutrophil percentage, lymphocyte percentage, N:L ratio, and behavioral measures. All variables in the model were fixed effects. The model included effects of trial, the three treatments, and two-way and three-way interactions. The level of significance used for testing the difference between treatment means was set at P < .05. The Proc Reg procedure of SAS was used to determine the relationship between number of stressors and hematologic and behavioral measurements.
Results
Aerial ammonia concentrations (measured once in each of Weeks 1 and 3) were low in both rooms used in this study (concentrations of ammonia averaged across the two trials for constant-thermoneutral and cycling-high temperature treatments were 2.5 +/- 0.25 ppm and 4.0 +/- 0.25 ppm, respectively). No hydrogen sulfide was detected.
Hematologic measures
Effects of treatments on hematologic measures are summarized in Table 1. There were no interactions among stressors, and main effect means are presented. Hematocrit did not differ among treatments (P > .05). Blood cortisol concentration after injecting dexamethasone was low and similar across treatments (Table 1), reflecting suppressed endogenous ACTH secretion (mimicking hypothalamic-pituitary-adrenal negative feedback). In addition, plasma-cortisol response under exogenous ACTH challenge was not affected by space-allowance treatment (P > .05; Table 1). However, there was an interaction between temperature and regrouping treatments (Table 1). When pigs were mixed at constant-thermo-neutral temperature, plasma cortisol did not change; however, it was higher after mixing in groups on the cycling-high temperature treatment (P < .05, Table 1).
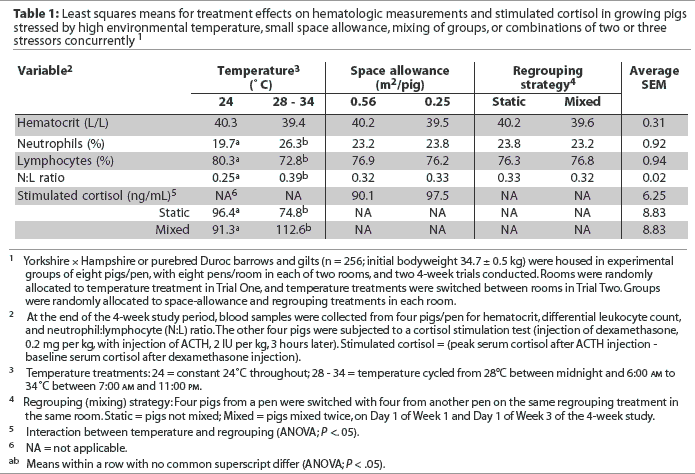
There was no effect of space or regrouping on percentages of neutrophils or lymphocytes or on N:L ratio (P > .05; Table 1). Heat stress resulted in a higher percentage of neutrophils and a lower percentage of lymphocytes, and, consequently, a higher N:L ratio (P < .05).
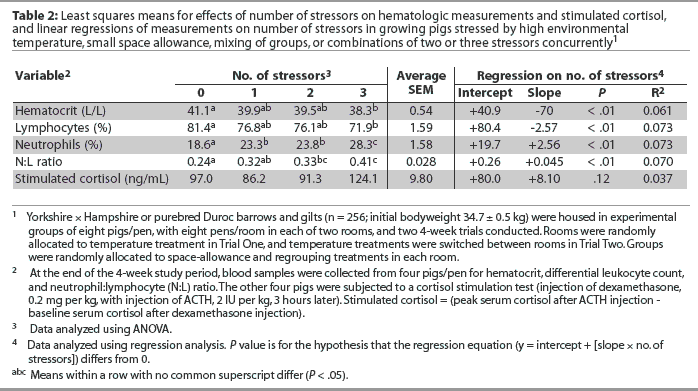
The effect of number of stressors on cortisol and hematalogic measures is summarized in Table 2. There was no effect (P > .05) of number of stressors on plasma cortisol concentrations (Table 2). Hematocrit was linearly lower as number of stressors increased from zero to three (P < .05). Percentage of lymphocytes was linearly lower, whereas percentage of neutrophils was linearly higher, as number of stressors increased (P < .05), resulting in a higher N:L ratio (P < .05) with increasing number of stressors (Table 2).
Behavioral measures
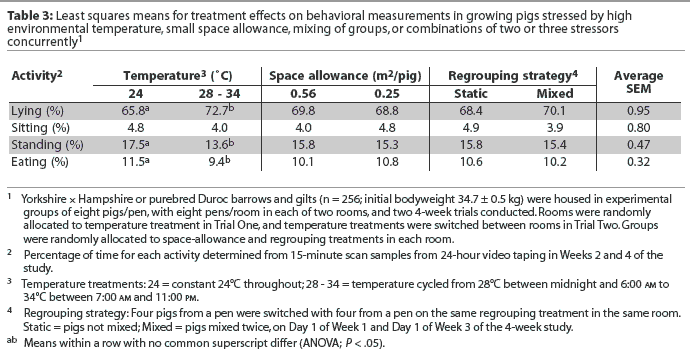
Pigs at cycling-high temperature had higher proportions of lying activity (P < .05) and lower proportions of standing and eating activities (P < .05) than did pigs at constant-thermoneutral temperature (Table 3).The space-allowance and regrouping stressors were not associated with changes in pigs’ lying, sitting, standing, or eating activities (Table 3).
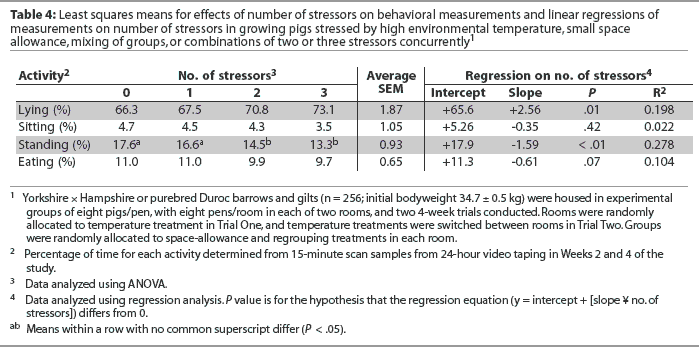
Relationships between number of stressors and behavior measures are summarized in Table 4. The regressions of lying and standing activity against number of stressors were significantly different from 0 and indicated that lying activity increased linearly and standing activity decreased linearly as the number of stressors increased from zero to three. In addition, there was a trend (P = .07) for eating activity to decrease with number of stressors. The regression of sitting activity on increasing number of stressors was not statistically different from 0 (P > .05; Table 4).
Diurnal patterns of behavior under the temperature treatments are illustrated in Figure 1. Space-allowance and regrouping treatments were not associated with a change in the diurnal pattern of behavior (P > .05). Percentage of pigs lying down at a particular time was lower (P < .05) at constant-thermoneutral temperature between 7:00 am and 1:00 pm, and at 6:00 pm and 12:00 midnight, than for the cycling-high temperature treatment (Figure 1A). A higher percentage (P < .05) of pigs were standing at constant-thermoneutral temperature between 7:00 am and 11:00 am and at 1:00 pm and 6:00 pm (Figure 1C). More feeder activity (P < .05) was observed in pigs at constant-thermoneutral temperature than in those in the cycling-high temperature environment between 7:00 am and 9:00 am, at 11:00 am and 1:00 pm, and at 9:00 pm, 11:00 pm, and 12:00 midnight (Figure 1D).
Discussion
The finding that mixing pigs resulted in higher plasma cortisol at cycling-high but not at constant-thermoneutral temperatures is in general agreement with results of chick studies that showed that plasma corticosterone was not affected by aerial ammonia, coccidiosis, electric shock, heat stress, or noise when these stressors were applied individually.8 High plasma cortisol concentration is related to high catabolic rate (low productive performance). Adrenal corticosteroids might be partly responsible for stress-associated immunosuppression. Adrenal hypertrophy, which tends to enhance adrenocortical response to ACTH, was found in pigs presumed to be chronically stressed.9 Cortisol concentration was also higher in tethered sows, plateauing 75 minutes after ACTH injection, but there was marked individual animal variation.9
Although there was no effect of space or regrouping on percentages of neutrophils or lymphocytes, or on N:L ratio, heat stress was associated with a higher N:L ratio; heat stress likewise resulted in a higher heterophil:lymphocyte (H:L) ratio in chicks.8,21 Higher N:L ratio is an indicator of a pig’s response to stress in controlled situations.17 Higher N:L ratio may also indicate a health challenge on the animal, reflecting a weakened immune system (and often, consequently, an animal in poor health). However, the pigs used in this study were from a herd negative for pocine reproductive and respiratory syndrome and were clinically healthy throughout the study. Pigs of intermediate social rank, as compared to dominant pigs, determined from outcomes of agonistic interactions, have lower absolute numbers of neutrophils, indicating less stress response, and socially dominant pigs tend to have higher absolute neutrophil levels relative to intermediates and subordinates.15 Relocation and regrouping resulted in lower pig growth rate and feed-conversion efficiency and increased signs of clinical disease, suggesting reduced immune competence.22 Results reported here and those of Hyun et al10 for growth performance in pigs in the same study, using the same treatments, showed that cycling-high temperature influenced both growth performance and a number of blood parameters associated with response to stress (ie, stimulated cortisol level, percentages of neutrophils and lymphocytes, and N:L ratio), whereas restricted space allowance and regrouping were associated with poorer growth performance but not with differences in blood parameters.
The lack of an effect of the number of concurrent stressors on plasma cortisol concentration in this study was similarly observed in research with chicks,8 and the increase in N:L ratio with increasing number of stressors is similar to the trend found for H:L ratio in chicks as number of stressors increased from zero to six.8 Linear relationships between number of stressors and blood parameter measurements suggest additive effects of increasing number of stressors in growing pigs for HCT, percentages of lymphocytes and neutrophils, and N:L ratio, although in the current study, the relationships between these blood parameter measures and number of stressors were weak (R2 < 0.1).
There has been discussion of the usefulness of blood parameters as quantitative indicators of stress. Ellersieck et al23 suggested that absolute and differential leukocyte counts were useful if they changed in rational ways when the animal was under stress. However, Riskowski et al24 suggested that complete blood count parameters, eg, hemoglobin, HCT, leukocyte count, plasma protein, fibrinogen, and N:L ratio, were too variable to be used in practical settings. Plasma cortisol concentration is highly correlated with N:L ratio. Widowski et al17 suggested that N:L ratio might be an indicator of the pig’s response to stress, and adrenocorticoids reduce proliferation of circulating lymphocytes and increase number of circulating neutrophils.25 Nevertheless, in the current study, changes in blood parameters with increasing number of stressors were modest.
In the current study, lying, sitting, standing, and eating activities were not affected by the space-allowance and regrouping stressors, but were affected by the environmental temperature treatment. In another study, the floor area used by the pigs for lying down increased with environmental temperature.24 Randolph et al3 also reported that space allowance did not affect percentages of time lying, sitting, standing, or eating in growing pigs, even though restricted space allowance increased aggressive behavior (eg, attack, threat, displacement at feeder). Spicer and Aherne26 reported no difference in percentages of time resting, active-nonfeeding, sitting, or biting in weanling pigs at different space allowances. Stookey and Gonyou27 observed that in finishing pigs, the percentage of time fighting increased until day 8 after regrouping, but percentages of time lying, standing, and eating, respectively, did not differ between static and mixed groups. The lesser amount of time spent eating by pigs on the cycling-high temperature treatment may help explain the reduction in growth rate in pigs exposed to this treatment.9 However, restricted space allowance and regrouping stressors did not influence animal behavior in this or in previous studies,3,26,27 even though growth performance was influenced by those stressors.10
Between 12:00 midnight and 6:00 am, although the environmental temperature was its lowest for the day, there was no effect of temperature treatment on the pigs’ diurnal feeding activity. Perhaps the cycling-high temperature environment had an effect on the activity peak during daytime. In previous studies carried out under thermoneutral conditions, pigs started eating at approximately 6:00 am to 7:00 am and highest feeder-related activity occurred between 9:00 am and 11: 00 am.28,29 In the current study, pigs on the cycling-high temperature did not show high feeding activity during the morning.
The regressions of lying and standing activity against number of stressors were significantly different from 0 and indicated that lying activity increased and standing activity decreased as number of stressors increased from zero to three, suggesting that pigs under stress reduce physical activity and thus spend less time standing and more time lying.
Overall, these results indicate that, in growing pigs, heat stress influenced certain blood parameter and behavioral measurements, and that some of these measurements were additively affected by multiple concurrent stressors (cycling-high temperature, low space allowance, and regrouping). It is postulated that the effects reported here could be used to estimate the total amount of stress associated with a pig’s residing in a specific, practical, multifactorial production environment. Further research is warranted in order to better understand effects of multiple concurrent stressors on the pig’s physiologic, behavioral, health, and performance measurements.
Implications
- The three stressors imposed in this study influenced blood parameter and behavioral measurements indicative of stress in growing pigs, with some effects additive.
- These additive effects of multiple concurrent stressors may partly explain why pigs in commercial units grow at a rate substantially below their genetic potential.
References
1. Close WH, Mount LE, Brown D. The effects of plane of nutrition and environmental temperature on the energy metabolism of the growing pig. 2. Growth rate, including protein and fat absorption. Br J Nutr. 1978;40:423-431.
2. Xin H, DeShazer JA. Feeding patterns of growing pigs at warm constant and cyclic temperatures. Trans ASAE. 1992;35:319-323.
3. Randolph JH, Cromwell GL, Stahly TS, Kratzer DD. Effects of group size and space allowance on performance and behavior of swine. J Anim Sci. 1981;53:922-927.
4. Lindemann MD, Kornegay ET, Meldrum JB, Schurig G, Gwazdauskas FC. The effect of feeder space allowance on weaned pig performance. J Anim Sci. 1987;64:8-14.
5. Kornegay ET, Meldrum JB, Chickering WR. Influence of floor space allowance and dietary selenium and zinc on growth performance, clinical pathology measurements and liver enzymes, and adrenal weights of weanling pigs. J Anim Sci. 1993;71:3185-3198.
6. McGlone JJ, Curtis SE. Behavior and performance of weanling pigs in pens equipped with hide areas. J Anim Sci. 1985;60:20-24.
7. Björk A, Olsson NG, Christensson E. Effects of amperozide on biting behavior and performance in restricted-fed pigs following mixing. J Anim Sci. 1988;66:669-675.
8. McFarlane JM, Curtis SE, Shanks RD, Carmer SG. Multiple concurrent stressors in chicks. 3. Effects on plasma corticosterone and heterophil:lymphocyte ratio. Poult Sci. 1989;68:522-527.
9. Janssens CJJG, Helmond FA, Wiegant VM. Increased cortisol response to exogenous adrenocorticotropic hormone in chronically stressed pigs: Influence of housing conditions. J Anim Sci. 1994;72:1771-1777.
10. Hyun Y, Ellis M, Riskowski GL, Johnson RW. Growth performance of pigs subjected to multiple concurrent environmental stressors. J Anim Sci. 1998;76:721-727.
11. McFarlane JM, Curtis SE, Shanks RD, Carmer SG. Multiple concurrent stressors in chicks. 1. Effects on weight gain, feed intake, and behavior. Poult Sci. 1989;68:501-509.
12. McGlone JJ, Lumpkin EA, Norman RL. Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J Anim Sci. 1993;71:888-896.
13. Kelley KW. Stress and immune function: a bibliographic review. Ann Res Vet. 1980;11:445-478.
14. Kelley KW. Immunological consequences of changing environmental stimuli. In: Moberg GP, ed. Animal Stress. Bethesda, Maryland: American Physiological Society; 1985:193-223.
15. Morrow-Tesch JL, McGlone JJ, Salak-Johnson JL. Heat and social stress effects on pig immune measures. J Anim Sci. 1994;72:2599-2609.
16. National Research Council. Nutrient Requirements of Swine. 9th ed. Washington, DC: National Academy Press; 1988.
17. Widowski TM, Curtis SE, Graves CN. The neutrophil:lymphocyte ratio in pigs fed cortisol. Can J Anim Sci. 1989;69:501-504.
18. von Borell E, Ladewig J. Relationship between behaviour and adrenocortical response pattern in domestic pigs. Appl Anim Behav Sci. 1992;34:195-206.
19. von Borell E, Ladewig J. Altered adrenocortical response to acute stressors or ACTH. Domest Anim Endocrinol. 1989;6:299-309.
20. Ladewig J, Smidt D. Behavior, episodic secretion of cortisol, and adrenocortical reactivity in bulls subjected to tethering. Horm Behav. 1989;23:344-360.
21. McKee JS, Harrison PC. Effects of supplemental ascorbic acid on the performance of broiler chickens exposed to multiple concurrent stressors. Poult Sci. 1995;74:1772-1785.
22. Hessing MJC, Tielen MJM. The effect of climatic environment and relocating and mixing on health status and productivity of pigs. Anim Prod. 1994;59:131-139.
23. Ellersieck MR, Veum TL, Durham TL, McVicker WR, McWilliams SN, Lasley JF. Response of stress-susceptible and stress-resistant Hampshire pigs to electrical stress. II. Effects on blood cells and blood minerals. J Anim Sci. 1979;48:453-458.
24. Riskowski GL, Bundy DS, Matthews JA. Huddling behavior and hematology of weanling pigs as affected by air velocity and temperature. Trans ASAE. 1990;33:1669-1675.
25. Moss BW, McMurray CH. The effects of the duration and type of stress on some serum enzyme levels in pigs. Res Vet Sci.1979;26:1-6.
26. Spicer HM, Aherne FX. The effects of group size/stocking density on weaning pig performance and behavior. Appl Anim Behav Sci. 1987;19:89-98.
27. Stookey JM, Gonyou HW. The effects of regrouping on behavioral and production parameters in finishing swine. J Anim Sci. 1994;72:2804-2811.
28. Hyun Y, Ellis M, Wilson ER, McKeith FK. Relationships between feed intake measurements, monitored using a computerized feed intake recording system, and growth performance and body composition of group-housed pigs. Asian-Aust J Anim Sci. 2000;13:1717-1725.
29. Hyun Y, Ellis M. Effect of group size and feeder type on growth performance and feeding patterns in growing pigs. J Anim Sci. 2001;79:803-810