Original research |
Peer reviewed |
Effects of type of machine and covering on viruses and microorganisms recovered from dust in ultrasound machines used in German swine production operations
Efectos del tipo de aparato y su cubierta en la recuperación de virus y microorganismos del polvo en los aparatos de ultrasonido utilizados en granjas de cerdos de Alemania
Effets du type de machine et sa couverture sur la récupération des virus et micro-organismes dans machines ultrasoniques utilisées dans les fermes porcines allemandes
Johannes Kauffold, Dr med vet; Jochen Beckjunker, Dr med vet; Regina Scheller, Dr med vet; Bernd-Andreas Schwarz, Dr med vet; Neville Beynon, MA, MCGI, FRAgS; Axel Sobiraj, DVM, MSc, PhD
JK, JB, AS: Large Animal Clinic for Theriogenology and Ambulatory Services, Faculty of Veterinary Medicine, University of Leipzig, Leipzig, Germany; RS, B-AS: Official Laboratory for Public and Veterinary Health Saxony, Leipzig, Germany; NB: Veyx Pharma UK, Charvil, Reading, UK; Corresponding author: Dr Johannes Kauffold, Large Animal Clinic for Theriogenology and Ambulatory Services, Faculty of Veterinary Medicine, University of Leipzig, An den Tierkliniken 29, 04103 Leipzig, Germany; Tel: (49)3419738364; Fax: (49)3419738398; E-mail: kauffold@rz.uni-leipzig.de.
Cite as: Kauffold J, Beckjunker J, Scheller R, et al. Effects of type of machine and covering on viruses and microorganisms recovered from dust in ultrasound machines used in German swine production operations. J Swine Health Prod. 2005;13(2):72-80.
Also available as a PDF.
SummaryObjectives: To investigate whether dust from the inside of ultrasound machines used for gynecological examinations in German swine production operations harbors viruses and microorganisms, and whether the type of machine and its covering affect internal viral and microbial contamination. Methods: Dust swabs were collected from 18 ultrasound machines of three common types. Five were used completely covered (closed plastic bags or household plastic cling-film), four incompletely covered (with open or perforated plastic bags), and nine uncovered. Swabs were tested for porcine circovirus type 2 (PCV-2) by polymerase chain reaction (PCR), for porcine reproductive and respiratory syndrome virus (PRRSV) by nested reverse transcriptase-PCR, and for bacteria, fungi, and yeasts by culturing. Eight new unused machines (two or three of each type) served as negative controls. Results: Neither PCV-2 DNA nor yeasts were recovered from any machine. Nine used machines, but no new machines, tested positive for PRRSV RNA. All used machines and two new machines harbored bacteria and fungi. Within the used machines, the type of machine did not affect contamination. Complete covering was most effective in reducing internal contamination with bacteria and fungi. Implications: During their use on swine farms, ultrasound machines of different types may become internally contaminated with the PRRSV and numerous microorganisms. A cover that would completely eliminate contact with air might be effective in preventing PRRSV contamination of these machines. Biosecurity procedures should be established for use of ultrasound machines on swine farms, including their complete covering with intact plastic bags. | ResumenObjetivos: Investigar si el polvo del interior de los aparatos de ultrasonido, utilizados para exámenes ginecológicos en las granjas de cerdos de Alemania, contenían virus y microorganismos, y si el tipo de aparato y su cubierta afectaban la contaminación interna viral y microbiana. Métodos: Con hisopos e recolectaron muestras de 18 aparatos de ultrasonido de tres tipos comunes. Cinco estaban completamente cubiertos (con bolsas de plástico cerradas o plástico adhesivo de de uso casero), cuatro estaban cubiertos de forma incompleta (con bolsas de plástico abiertas o perforadas), y nueve estaban descubiertos. Los hisopos fueron examinadas en busca del circovirus porcino tipo 2; (PCV-2 por sus siglas en inglés), mediante la reacción en cadena de la polimerasa (PCR por sus siglas en inglés), del virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) a través del PCR de trascripción reversa anidado y de bacterias, hongos y levaduras a través de cultivo. Ocho aparatos nuevos y sin usar (dos o tres de cada tipo) sirvieron como controles negativos. Resultados: Ni el DNA del PCV-2 ni de las bacterias se recuperaron de ninguna máquina. Nueve aparatos fueron positivos al RNA del PRRSV, sin embargo, ningún aparato nuevo fue positivo. Todos los aparatos usados y dos de los aparatos nuevos tenían bacterias y hongos. Dentro de la categoría de aparatos usados, el tipo de aparato no afectó la contaminación. El cubrir a los aparatos completamente fue el tratamiento más efectivo para reducir la contaminación interna con bacterias y hongos. Implicaciones: Las máquinas de ultrasonido de diferentes tipos pueden contaminarse internamente con el PRRSV y con numerosos microorganismos durante su uso en las granjas de cerdos. Una cubierta que elimine completamente el contacto con el aire podría ser efectiva para prevenir la contaminación con PRRSV de estos aparatos. Se deben establecer procedimientos de bioseguridad para el uso de aparatos de ultrasonido en granjas de cerdos, incluyendo su cubierta total con bolsas de plástico intactas. | ResuméObjectifs: Enquêter si la poussière a l’interior de machines ultrasoniques utilisées pour les examens gynécologiques dans les fermes porcines allemandes héberge des virus et des micro-organismes, et si le type de machine et sa couverture affectent la contamination interne virale et microbienne. Méthodes: Écouvillons avec la poussière ont été rassemblées de 18 machines ultrasoniques de trois types communs. Cinq ont été utilisés complètement couvert (sacs plastiques fermés ou film plastique), quatre couvert incomplètement (avec sacs plastiques ouverts ou perforés), et neuf à découvert. Les écouvillons ont été testés pour circovirus porcin type 2 (PCV-2 par ses initiales en anglais) par amplification en chaîne par polymérase (PCR par ses initiales en anglais), pour le virus du syndrome reproducteur et respiratoire porcin (PRRSV par ses initiales en anglais) par n-PCR par transcriptase inverse, et pour bactéries, fungique, et levures par une culture. Huit machines nouvelles inutilisées (deux ou trois de chaque type) ont servi comme contrôles négatives. Résultats: Ni l’ADN du PCV-2 ni levures n’ont été récupérés de aucune machine. Neuf machines usagées, mais aucune machine nouvelle, ont testé positif pour l’ARN de PRRSV. Toutes les machines usagées et deux machines nouvelles ont hébergé des bactéries et des fungiques. Dans les machines usagées, le type de machine n’a pas affecté la contamination. Le revêtement complet était très efficace pour réduire la contamination interne avec bactéries et fungiques. Implications: Les machines ultrasoniques de types différents peuvent être contaminées intérieurement avec le PRRSV et nombreux micro-organismes pendant leur usage sur les fermes porcines. Une couverture qui éliminerait complètement le contact avec l’air peut être efficace pour prévenir la contamination de PRRSV de ces machines. Des procédures de biosécurité devraient être établies pour l’usage de machines ultrasoniques sur fermes porcines, en comprenant leur revêtement complet avec les sacs du plastique intacts. |
Keywords: swine, ultrasound, ultrasound
machines, contamination, biosecurity
Search the AASV web site
for pages with similar keywords.
Received: January
28, 2004
Accepted: June
18, 2004
Ultrasonography has been successfully used for about a decade for routine pregnancy diagnosis in swine.1-3 Demonstration that ultrasonography is also appropriate for visualizing the ovaries and uterus of nonpregnant female pigs (for instance to monitor ovulation,4 to examine females with reproductive failure,5 or to determine sexual maturity in gilts),6 additionally facilitated its increasing use in swine production operations. According to the number of machines sold in Germany during the last 10 years, it is estimated that more than 400 ultrasound machines are in use by approximately 300 different institutions involved with swine production (eg, government agencies, universities, veterinary practices, and commercial swine production operations). Appropriate machines (approximately 10 types) are more or less compact and transportable, can be run without an external power supply, and have external surfaces that are reasonably easy to clean and disinfect. Most machines are equipped with fans to avoid overheating, but there is nothing to prevent dust and potential pathogens from moving in and out with the air moved by the fan. Because individual machines are commonly used in more than one herd daily, some users cover the machines prior to entering the farms, suggesting that this reduces or prevents internal contamination with swine pathogens and their spread from one herd to another. However, others do not use covers for various reasons eg, reduced image quality through increasing light reflection, transducer and cables uncovered in any case. Whether there is a real risk that ultrasound machines might be involved in aerosol transmission of swine pathogens is unknown, and it is currently unproven that this can be prevented using covers. As a first step towards clarifying these issues, the current study was conducted to investigate whether dust from the inside of ultrasound machines used in German swine production operations harbors viruses and other microorganisms (bacteria, fungi, yeasts), and whether the type of machine and cover affects internal viral and microbial contamination.
Materials and methods
Ultrasound machines
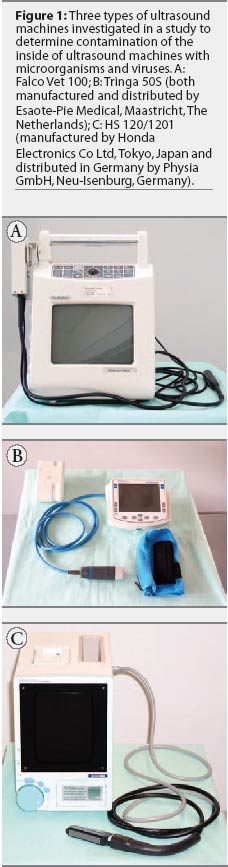 A total of 26 ultrasound machines of three types commonly used in German
swine production operations were investigated in this study. Eighteen machines had been
in use prior to sampling. The used machines, illustrated in Figure 1, were of three
types: Falco Vet 100 (n = 6) and Tringa 50S (n =
4), which are both manufactured and distributed by Esaote-Pie Medical,
Maastricht, The Netherlands; and HS 120 and the
new model HS 1201 (n = 8), manufactured by Honda Electronics Co Ltd, Tokyo,
Japan and distributed in Germany by Physia GmbH, Neu-Isenburg, Germany. The
remaining eight machines (three Falco Vet 100, three HS 120/1201, and two
Tringa 50S) were new and unused and served as negative controls. The used
machines tested were from one university, nine veterinary practices, and three
government agencies, located in eight German
federal states (Bavaria, Lower Saxony,
Mecklenburg-Western Pomerania, North
Rhine-Westphalia, Saxony, Saxony-Anhalt,
Schleswig-Holstein, and Thuringia). The unused machines
were tested at Esaote-Pie Medical in Maastricht (Falco Vet 100, Tringa 50S) and at
Physia GmbH in Neu-Isenburg (HS 120/1201).
A total of 26 ultrasound machines of three types commonly used in German
swine production operations were investigated in this study. Eighteen machines had been
in use prior to sampling. The used machines, illustrated in Figure 1, were of three
types: Falco Vet 100 (n = 6) and Tringa 50S (n =
4), which are both manufactured and distributed by Esaote-Pie Medical,
Maastricht, The Netherlands; and HS 120 and the
new model HS 1201 (n = 8), manufactured by Honda Electronics Co Ltd, Tokyo,
Japan and distributed in Germany by Physia GmbH, Neu-Isenburg, Germany. The
remaining eight machines (three Falco Vet 100, three HS 120/1201, and two
Tringa 50S) were new and unused and served as negative controls. The used
machines tested were from one university, nine veterinary practices, and three
government agencies, located in eight German
federal states (Bavaria, Lower Saxony,
Mecklenburg-Western Pomerania, North
Rhine-Westphalia, Saxony, Saxony-Anhalt,
Schleswig-Holstein, and Thuringia). The unused machines
were tested at Esaote-Pie Medical in Maastricht (Falco Vet 100, Tringa 50S) and at
Physia GmbH in Neu-Isenburg (HS 120/1201).
Historical data
All collaborating institutions were requested to provide relevant historical data for the used machines, identified individually in Table 1. All used machines had been in use for 0.5 to 8 years, and the total number of swine production units visited prior to this study ranged from three to 100 per machine. Except for one machine (number 3), all were frequently used in at least two herds per day, with a maximum of six herds daily.
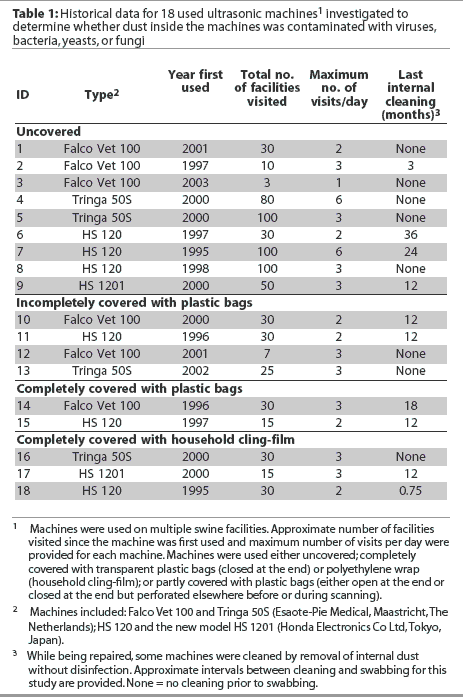
Five machines were always completely covered during scanning, either with plastic bags (n = 2) or with polyethylene wrap (household cling-film; n = 3), as illustrated in Figure 2. The plastic bags were transparent and disposable, but more detailed information was not available. Machines were oriented with the screens at the blind end of the bags, and the open ends of the bags were then closed with plastic strips or string. When cling-film was used, it was overlapped to completely cover the machines. Irrespective of the materials and methods used for complete covering, the transducer and cables remained uncovered. For two machines covered with closed plastic bags (numbers 12 and 13), it was reported that the bags were frequently torn or were intentionally perforated to facilitate handling prior to or during scanning. These machines were categorized as incompletely covered machines, and together with two machines covered with open plastic bags (numbers 10 and 11), they accounted for a total of four incompletely covered units. The remaining nine machines were used without covers.
Ten machines had been dismantled for repairs 3 weeks to 3 years prior to sampling, and at that time, the inside of each machine had been cleaned using compressed air. The machines were not disinfected inside, and were returned to use immediately following these repairs. Approximately equal percentages of these ten machines were categorized as completely covered, incompletely covered, or uncovered (Pearson´s chi-square test, P > .05 ), and as none of them had been cleaned or disinfected inside at any other time since they had been in use, the single cleaning that they had received was considered of no significance to interpretation of the results. Collaborators were asked whether any visited herds were positive (clinically, serologically, or both) for porcine reproductive and respiratory syndrome virus (PRRSV) or porcine circovirus type 2 (PCV-2; the agent of postweaning multi-systemic wasting syndrome). Some herds were reported positive for both; however, no information was given for the total number of PRRSV-positive and PCV-2-positive herds in which each of the machines was used.
Swabbing
For each machine, four sterile swabs were provided, two with Stuart medium (Heipha-Biotest, Dreieich, Germany) for microbiological culture and two for virus isolation (Medical Wire and Equipment Co Ltd, Corsham, UK). For each viral swab, tubes containing 2 mL of a transport medium (PBS supplemented with antibiotics) were supplied, to be added to the tubes containing the swabs after the specimens were taken. Guidelines for taking the swabs were provided to standardize the procedures.
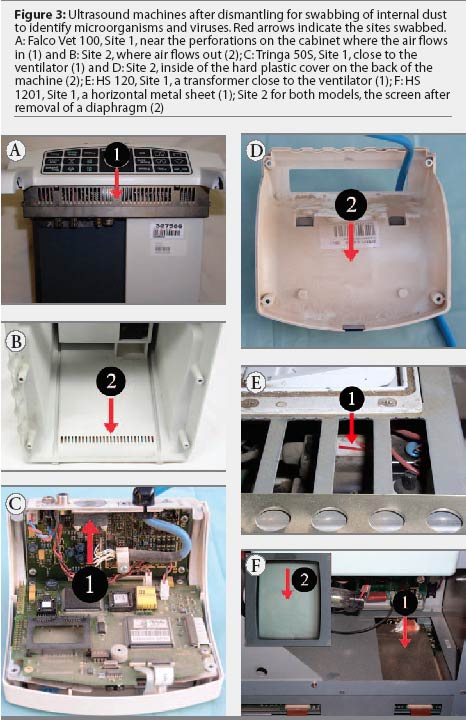
The ultrasound machines were dismantled for swabbing. Two dust swabs, one each for microbiological culture and virus isolation, were collected at two different but defined sites in each machine (Figure 3). The sizes of the sampled areas were not standardized. The swabs for virus isolation were first moistened with transport medium. Swabs for microbial culture and for virus isolation were rotated repeatedly at each site in order to sample a similar and sufficient amount of dust. Swabs were immediately refrigerated and submitted to the Official Laboratory for Public and Veterinary Health Saxony by overnight delivery service, and processed immediately after arrival.
Detection of viruses
The PRRSV and PCV-2 were chosen as indicator pathogens to provide information on whether dust from the insides of the ultrasound machines contained viruses. After each virus swab was collected, it was pressed and then rotated against the inside of the tube containing the 2 mL of transport medium, and the swab was then discarded. Total RNA was extracted from a 200-mL aliquot of the transport medium using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A multiplex nested reverse transcriptase-polymerase chain reaction (nRT-PCR) was used to detect PRRSV RNA, as described previously.7 A further 200-mL aliquot of the transport medium was used for DNA extraction using the Invisorb Spin Tissue Mini Kit (Invitek, Berlin, Germany) according to the manufacturer’s instructions. For PCV-2 analysis, 5 mL of the resulting DNA was tested by PCR as previously described.8 A machine was considered positive for PRRSV or PCV-2 if at least one of the two dust specimens tested positive for the respective viruses.
Detection of bacteria, fungi, and yeasts
For isolation of bacteria, swabs were inoculated on Gassner agar (Sifin, Berlin, Germany) and on blood-dextrose agar with 5% bovine blood, both directly and after an 18-hour to 24-hour enrichment culture in dextrose meat broth. For isolation of fungi and yeasts, swabs were inoculated on Sabouraud agar (Sifin). Inoculated agars and broth were cultured both under aerobic and micro-aerophilic conditions at 37°C for 24 to 72 hours. Bacteria were pre-identified according to colony morphology, hemolysis, and the results of Gram staining. Isolates were then subcultured and finally differentiated by characterization of biochemical and serological properties. The degree of contamination with microorganisms was estimated according to growth rate and number of colonies, and defined as follows: 1 = growth after enrichment culture; 2 = <= 10 colonies, 3 = 11 to 25 colonies, and 4 = > 25 colonies after direct culture.
Statistical analysis
Statistical analysis was performed in SPSS (SPSS GmbH, Munich, Germany) and included comparison of previous use of the machine, type of machine, and covering procedure as determinants. Results of virus detection were analysed using Pearson´s chi-square test. For each machine, the total number of species of bacteria, fungi, and yeasts (total isolated from both sites swabbed) and the categorical degree of contamination (average for both sites swabbed) were compared using the Mann-Whitney U test. Level of significance was set at P = .05 for all statistical tests.
Results
Viral and microbial contamination in used and unused ultrasound machines
All unused and used machines tested negative for PCV-2 DNA. None of the unused machines, but nine used machines, tested positive for PRRSV RNA at one (n = 7) or both (n = 2) swab sites (Table 2; P < .05), and either for the European strain alone (n = 8) or for the European strain plus the North American strain (n =1; Table 3). The mean number of species of microorganisms and mean categorical degree of contamination were higher for used than for unused machines (Table 2; P < .001). No yeasts were cultured from any of the unused or used machines. No microorganisms were cultured from six of the unused machines. The remaining two unused machines harbored a total of two gram-positive species (Bacillus species and coagulase-negative Staphylococcus species). In contrast, microorganisms were cultured from each of the used machines, for a total of 10 bacterial species (six gram-positive and four gram-negative) and two fungi (Table 3).
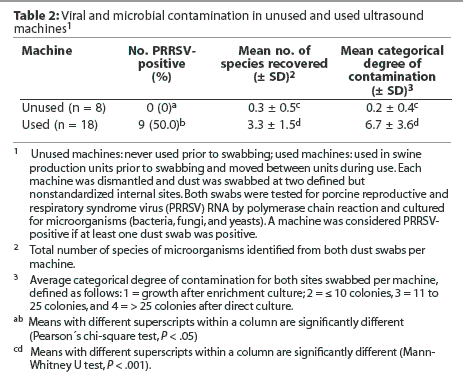
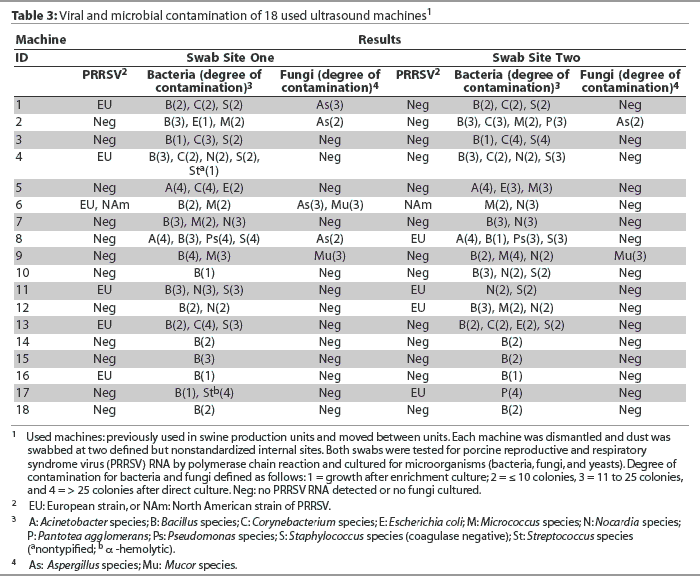
Effect of type of machine and type of covering on viral and microbial contamination in used ultrasound machines
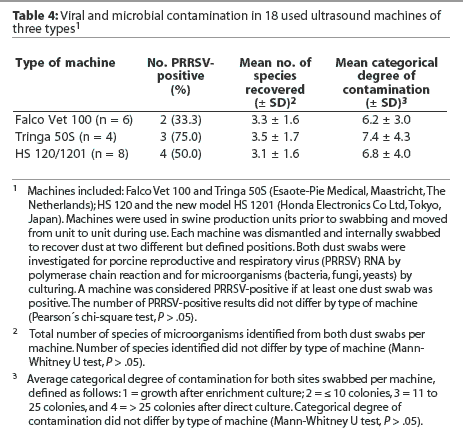
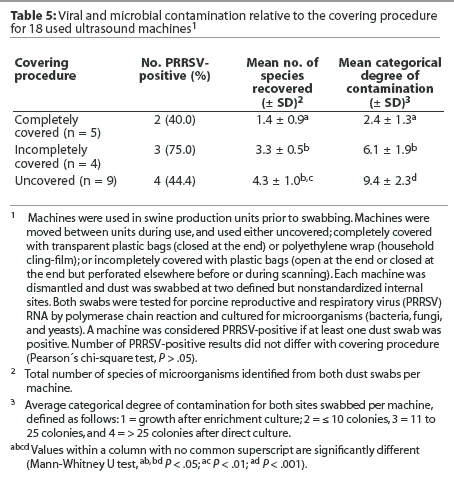
Within the used machines, the type of machine was not associated with differences in the percentage of PRRSV-positive results or in the mean number of species of microorganisms and mean categorical degree of contamination (Table 4). Similarly, the type of covering was not associated with differences in internal contamination with PRRSV (Table 5). However, it should be noted that among the three machines completely covered with cling-film, two tested positive for PRRSV RNA (numbers 16 and 17). In addition, machines numbered 12 and 13, which were categorized as incompletely covered because the closed plastic bags used for covering were frequently perforated prior to or during scanning, were also PRRSV-positive (Table 3). The mean number of species of microorganisms was lower for completely covered machines than for incompletely covered machines (P < .05) and uncovered machines (P < .01), and the mean categorical degree of contamination was lower for completely covered machines than for incompletely covered machines (P < .05) and uncovered machines (P < .001; Table 5).
Discussion
This study demonstrates that dust from the inside of ultrasound machines used in swine production operations may contain RNA from North American or European strains of PRRSV or both. It is most likely that contamination of the machines occurred via the air, probably facilitated by the action of the ventilator fans, since all of the used machines tested in this study were protected against internal contact with potential virus-containing materials (eg, feces, urine, saliva) by covers, their cabinets, or both. Additional support for this assumption comes from the results of three recent experiments demonstrating that the PRRSV can be transmitted as an aerosol, not only over short, but also longer distances.9-11 Moreover, it is also likely that the source of contamination was the air of the infected swine environment, since all unused machines were PRRSV-negative. This study failed to detect DNA of PCV-2 in any of the used machines tested, suggesting that internal contamination of the machines via air did not occur. It is unknown whether or not PCV-2 is transmitted by air; to our knowledge, there is only one recent epidemiological report by Danish practitioners suggesting that wind transmission may be possible.12
A total of 10 bacterial species and two fungal species were isolated from used machines, corresponding well in number and diversity to those found in air samples recovered from different swine confinement facilities.13-16 This suggests that the microorganisms had entered the machines via the air, and moreover, that the microorganisms had originated primarily from air contaminated by animals, which is strongly supported by the fact that both the mean number of species of microorganisms and the mean categorical degree of contamination per machine were much higher for used than for unused machines. Whether the microorganisms detected inside the machines included swine pathogens was not determined. However, it is of concern, for example, that one machine (number 17) harbored a high number of an [alpha]-hemolytic streptococcus, which might have been the swine pathogen Streptococcus suis, an organism that is transmitted as an aerosol under research conditions.17
Collectively, the results of this study suggest that internal viral and microbial contamination of ultrasound machines used in swine production facilities occurred via the air. However, this study did not answer the question of whether ultrasound machines may serve as vectors for swine pathogens, specifically for PRRSV.
Firstly, we did not show whether PRRSV found inside the machines was viable and infectious, since the virus was not isolated and a swine bioassay not performed. As has been shown in previous studies, survivability of PRRSV outside the host is very low. For example, PRRSV remains viable on inanimate materials at 20 degrees C for only a few hours (eg, 0.5 to 2 hours on metal and 2 to 8 hours on plastic).18,19 As PRRSV is thermally labile,20 and considering that the temperatures that developed inside the HS 120 and HS 1201 models at the end of a 2-hour run were 33°C and 36°C, respectively (as estimated in two replicated measurements at room temperature; data not shown), the probability that the virus survives inside the machines for a longer time is relatively low. Although this reduces to a minimum the risk of carrying live viruses from one swine production unit to another in ultrasound machines, it might nevertheless occur. The machines numbered 4 and 7, for example, were frequently used on six swine production units per day (ie, during an interval of approximately 12 hours, with approximately 2 hours between visits). In our experience, visiting more than one swine facility within a few hours is not an exception. However, a final answer to the question on the survivability of PRRSV inside the machines requires an experimental study in which machines are artificially inoculated and subsequently investigated for live viruses at defined intervals.
Secondly, we did not investigate whether swine pathogens are expelled from inside the machines into the environment and then transmitted to susceptible swine. However, if one assumes that the insides of the ultrasound machines become contaminated via air, then it might be possible that a retrograde spread of pathogens does occur.
Even though several unanswered questions are left by this study in demonstrating that ultrasound machines act as vectors for swine pathogens, detection of PRRSV and microorganisms inside the ultrasound machines per se emphasizes a current need for caution when using ultrasound equipment in different swine production units and especially when visits to different facilities occur within short intervals on the same day.
Whatever type of machine was used, this study demonstrated that the insides of ultrasound machines harbored PRRSV (as detected by the presence of specific RNA), bacteria, and fungi. Since this was probably facilitated through the action of the ventilator fan, it suggests that other types of machines equipped with fans and used in an infected environment might also become contaminated.
In this study, contamination with bacteria and fungi was lowest in machines that were completely covered during scanning. While this suggests that complete covering reduces internal microbial contamination, failure to designate the size of a standardized swabbing site in this study may have resulted in a sampling bias. In contrast to microorganisms, PRRSV contamination occurred irrespective of the procedure used for covering. Considering the possibility that PRRSV is spread as an aerosol, the fact that dust from the inside of incompletely covered and uncovered machines contained PRRSV RNA was not surprising. For the same reason, however, it was astonishing that two of the five completely covered machines also tested positive for PRRSV RNA. One explanation might be that whatever covers are used, they are ineffective in preventing PRRSV contamination. However both of the PRRSV-positive, completely covered machines belonged to the group of three machines covered with household cling-film, suggesting that the cling-film was not as effective as necessary in preventing viral contamination, possibly because some parts of the machines remained uncovered. A similar lack of protection against viral contamination may be assumed for PRRSV-positive machines 12 and 13, which were incompletely covered with closed plastic bags. In these two machines, the covers were either accidentally or intentionally perforated prior to or during scanning, suggesting that aerosol contamination might have occurred via the perforation. Therefore, it might be suggested that only covers that completely eliminate contact with air potentially containing PRRSV are effective in preventing PRRSV contamination, and that this may be achieved by use of intact and closed plastic bags. Such covers would then also reduce or prevent external contamination of the machines, thus making external cleaning and disinfection easier and more effective.
One limitation of this study in evaluating the effect that type of machine and covering have on internal viral and microbial contamination is the small number of machines in some categories. Whether this had an influence on the results remains questionable; however, the differences or similarities in viral and microbial parameters between groups were pronounced in most instances. The effect of different covers on PRRSV contamination should be further investigated under standardized conditions, possibly using larger and equal group sizes, which will probably only be achieved under controlled research conditions. However, before such a study is conducted, the role that ultrasound machines may or may not play in the spread of PRRSV and swine pathogens in general should be investigated. This will clarify the need to cover or encase ultrasound machines. Redesigning machines to prevent internal contamination, thereby reducing the risk of their acting as vectors for swine pathogens, would be beneficial. For instance, it would be of tremendous help if the machines could be run without ventilator fans. However, until we have answers and solutions to all of the open issues, users may consider adapting the following recommendations as minimum biosecurity requirements.
Completely cover the ultrasound machine with sturdy transparent disposable plastic bags before entering a swine production unit. Check with the supplier to determine whether this would be detrimental to the machines (ie, whether it would cause overheating). Clean and disinfect the machine externally, including cables and transducers, before entering the next swine production unit. Finally, avoid using the machine in different swine production facilities within short intervals during the same day.
Implications
- Dust inside ultrasound machines may be contaminated with microorganisms that threaten biosecurity when the machines are used in different herds.
- Completely covering ultrasound machines with plastic bags or household cling-film reduces internal contamination with bacteria and fungi.
- Completely covering machines with intact plastic bags might also prevent contamination with PRRSV.
- The role of ultrasound machines in aerosol transmission of swine pathogens, especially of PRRSV, should be investigated.
- Redesigning ultrasound machines would improve their biosecurity properties.
- Minimum biosecurity recommendations include covering ultrasound machines completely with intact disposable plastic bags before entering a swine production unit, cleaning and disinfecting the machines externally after each visit, and avoiding use of the machines in different swine production facilities within short intervals during the same day.
Acknowledgements
The authors thank Jürgen Eberspächer (Physia GmbH, Neu-Isenburg, Germany; supplier of the HS 120/1201) for having the courage to support this study logistically and financially. We are grateful to Frank Kemme (Esaote-Pie Medical, Maastricht, The Netherlands) and to all practitioners and officials who donated their time and skill in taking the swabs and sending them to Leipzig. Thanks also to Andreas Richter (Large Animal Clinic for Theriogenology and Ambulatory Services, Faculty of Veterinary Medicine, University of Leipzig) for performing the statistical analysis.
References
1. Knox RV, Althouse GC. Visualizing the reproductive tract of the female pig using real-time ultrasonography. Swine Health Prod. 1999;7:207-215.
2. Kauffold J, Richter A, Sobiraj A. [Results and experiences of pregnancy control in swine by ultrasonography on different days of gestation over two years.] Tierärztl Prax. 1997;25:429-437.
3. Martinat-Botté F, Renaud G, Madec F, Costiou P, Terqui M. Ultrasonography and Reproduction in Swine. Paris, France: INRA Editions; 1998.
4. Weitze KF, Habeck O, Willmen T, Rath D. Detection of ovulation in the sow using transcutaneous sonography. Zuchthygiene. 1989;24:40-42.
5. Kauffold J, Rautenberg T, Gutjahr S, Richter A, Sobiraj A. Ultrasonographic characterization of the ovaries in non-pregnant first served sows and gilts. Theriogenology. 2004;61:1407-1417.
6. Kauffold J, Rautenberg T, Richter A, Waehner M, Sobiraj A. Ultrasonographic characterization of the ovaries and the uterus in prepubertal and pubertal gilts. Theriogenology. 2004;61:1635-1648.
7. Gilbert SA, Larochelle R, Magar R, Cho HJ, Deregt D. Typing of porcine reproductive and respiratory syndrome viruses by a multiplex PCR assay. J Clin Microbiol. 1997;35:264-267.
8. Morozov I, Sirinarumitr T, Sorden SD, Halbur PG, Morgan MK, Yoon KJ, Paul PS. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J Clin Microbiol. 1998;36:2535-2541.
*9. Kristensen CS, Botner A, Angen O, Sorensen V, Jorsal SE, Takai H, Barfod K, Nielsen JP. Airborne transmission of A. pleuropneumoniae and PRRS virus between pig units. Proc 17th IPVS Congress. Ames, Iowa. 2002:272.
10. Brockmeier SL, Lager KM. Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Vet Microbiol. 2002;89:267-275.
*11. Dee S, Jacobson L, Rossow K, Pijoan C. Developing a model to re-evaluate aerosol transmission of PRRSV. Proc 4th Int Symp Emerging Re-emerging Pig Dis. Rome, Italy. 2003:39-40.
*12. Rathkjen PH, Condradsen P, Nielsen E, Riising H-J. Spread of PMWS within an area of Denmark – an epidemiological report from practice. Proc 4th Int Symp Emerging Re-emerging Pig Dis. Rome, Italy. 2003:175-176.
13. Mackiewicz B. Study on exposure of pig farm workers to bioaerosols, immunologic reactivity and health effects. Ann Agric Environ Med. 1998;5:169-175.
14. Andersson AM, Weiss N, Rainey F, Salkinoja-Salonen MS. Dust-borne bacteria in animal sheds, schools and children´s day care centres. J Appl Microbiol. 1999;86:622-634.
15. Zucker B-A, Trojan S, Müller W. Airborne gram-negative bacterial flora in animal houses. J Vet Med B. 2000;47:37-46.
16. Chang CW, Chung H, Huang CF, Su HJJ. Exposure of workers to airborne microorganisms in open-air swine houses. Appl Environ Microb. 2001;67:155-161.
17. Berthelot-Herault F, Gottschalk M, Labbe A, Cariolet R, Kobisch M. Experimental airborne transmission of Streptococcus suis capsular type 2 in pigs. Vet Microbiol. 2001;82:69-80.
18. Dee S, Deen J, Rossow K, Wiese C, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during cold weather. Can J Vet Res. 2002;66:232-239.
19. Dee S, Deen J, Rossow K, Wiese C, Eliason R, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during warm weather. Can J Vet Res. 2003;67:12-19.
20. Benfield DA, Collins JE, Dee SA, Halbur PG, Joo HS, Lager KM, Mengeling WL, Murtaugh MP, Rossow KD, Stevenson GW, Zimmerman JJ. Porcine reproductive and respiratory syndrome. In: Straw BE, D´Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:201-232.
* Non-refereed references.