Original research |
Peer reviewed |
The impact of ground water high in sulfates on the growth performance, nutrient utilization, and tissue mineral levels of pigs housed under commercial conditions
John F. Patience, PhD; A. Denise Beaulieu, PhD; Doug A. Gillis
Prairie Swine Centre Inc, Saskatoon, Saskatchewan, Canada. Corresponding author: Dr A. Denise Beaulieu, Prairie Swine Centre Inc, PO Box 21057, 2105 8th St E, Saskatoon, Saskatchewan, Canada S7H 5N9; Tel: 306-667-7441; Fax: 306-955-2510; E-mail: beaulieu@sask.usask.ca
Cite as: Patience JF, Beaulieu AD, Gillis DA. The impact of ground water high in sulfates on the growth performance, nutrient utilization, and tissue mineral levels of pigs housed under commercial conditions. J Swine Health Prod. 2004;12(5):228-236.
Also available as a PDF.
Summary
Objective: To examine the effect of drinking water high in sulfates on the growth performance, nutrient utilization, and tissue mineral levels of newly weaned pigs housed in a commercial operation.
Materials and methods: Performance and nutrient digestibility were determined for pigs consuming either water treated by reverse osmosis (RO; 219 mg total dissolved solutes (TDS) per L, 29 mg sulfates per L) or untreated ground water (3078 mg TDS per L, 1650 mg sulfates per L). In each of two experiments, approximately 240 pigs (20 +/- 3 days of age, 5.9 +/- 1.0 kg) received either RO-treated or untreated water for 35 days. In Experiment One, water was delivered either via nipple drinkers or dish-type drinkers. In Experiment Two, diets either contained supplemental zinc oxide (3000 mg zinc per kg) or no supplemental zinc.
Results: Average daily gain, average daily feed intake, gain-to-feed ratio, water disappearance, and nutrient digestibility were similar regardless of water source (P > .05). Water disappearance was lower (P < .01) and feed efficiency tended to be higher (P < .10) when pigs used dish-type drinkers. Average daily gain and feed intake were lowest when pigs consumed a diet with added zinc (P < .05) and, for the first 3 weeks after weaning, when they also received untreated water (diet-by-water interaction; P < .05).
Implications: Weanling pigs can tolerate drinking water containing high concentrations of sulfates. Poor performance or diarrhea in nursery pigs should not be attributed to water quality until other possible contributing factors are investigated.
Keywords: swine, water
quality, sulfates, reverse osmosis, tissue minerals
Search the AASV web site
for pages with similar keywords.
Received: October
14, 2003
Accepted: December
16, 2003
An adequate quantity of potable drinking water is a necessity for successful pork production. One indicator of water quality is the concentration of total dissolved solids (TDS). Appropriate concentrations of TDS in drinking water for swine have not been well defined. The Task Force on Water Quality Guidelines1 recommends a maximum of 3000 mg TDS per L. However, the Nutrient Requirements for Swine2 has much broader guidelines, stating only that water containing 1000 mg TDS per L is safe to use, while water containing > 7000 mg TDS per L should not be offered to swine for consumption. Sulfate salts are known for their laxative effects and are a common contributor to water TDS. The recommended maximum sulfate level in drinking water for swine has been set at 1000 mg per L.1 A survey of well water used on swine farms in Saskatchewan found that 7.4% of wells contained > 3000 mg TDS per L, and 25% contained > 1000 mg sulfates per L.3 Producers often attribute diarrhea and poor performance in weanling pigs to water quality problems; research, however, does not substantiate this conclusion.4,5
Some of the variation in response to water quality may be due to other management factors that influence the susceptibility of the pig to diarrhea, or to the gastrointestinal disturbance associated with sulfate-induced osmotic diarrhea. When pigs consumed water containing either 217 or 4390 mg TDS per L (83 or 2650 mg sulfates per L, respectively), ADG was lower for pigs that consumed the poor quality water, but only when the diet was free of antibiotics.4 Supplementing weanling pigs’ diet with pharmacological levels of zinc usually results in improved ADG,6,7 possibly because postweaning diarrhea is controlled.8 Thus, a high concentration of dietary zinc may be one factor mitigating the effect of poor quality water.
The overall objective of our study was to determine the impact on ADG, average daily feed intake (ADFI), nutrient digestibility, and tissue mineral concentrations when pigs housed under commercial conditions consume water high in sulfates. A decrease in nutrient or feed dry matter digestibility implies an increased rate of passage and was thus used as an indication of diarrhea. Mineral concentrations in tissues and serum were measured to determine whether excess mineral intake from the drinking water accumulated in specific tissues. Other serum chemistry measurements were obtained to allow an estimation of the effect of water quality on overall health of the pigs. For example, an increase in blood proteins, specifically albumin, is indicative of dehydration, which would be expected if the pigs were suffering from diarrhea.
Materials and methods
Facilities
Two experiments were conducted at a 1200-sow farrow-to-finish commercial farm located in central Saskatchewan. Pigs were managed according to standard farm practice. Room temperature, ventilation, and humidity levels were monitored daily. The initial temperature in the nursery was 28°C; this was lowered by approximately 1.0 C° per week after weaning. The lighting schedule was maintained at 15 hours of light and 9 hours of dark.
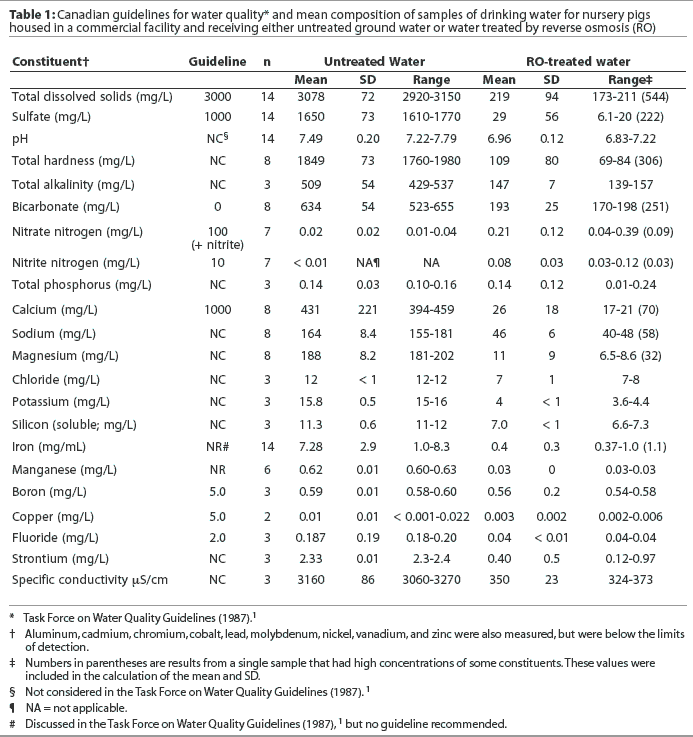
Water on the farm was obtained from a deep well and was treated by a reverse osmosis (RO) system prior to delivery to the herd. The RO-treated water was stored in a concrete cistern and then delivered to the pigs. For the purposes of this experiment, a separate water delivery system was installed to allow the performance of pigs consuming untreated water to be compared to that of pigs consuming the RO-treated water. A pump and pressure system was installed to deliver untreated water directly from the well to the specified pens. Water composition is presented in Table 1.
One nursery consisting of 28 pens was used in both experiments. Two pens at the end of the room were left vacant to provide room for experimental equipment, leaving 26 pens to house the animals on test (approximately 10 pigs per pen). Drinking water treatments were assigned to alternate pairs of pens, with one unpaired pen in each treatment (13 pens per water treatment). The barn was equipped with nipple drinkers in each pen. Because these are prone to spillage, dish-type drinkers were installed (Drik-O-Mat water cups; Prairie Pride Enterprises, Winnipeg, Manitoba). Within each water treatment, half of the pairs of pens were randomly assigned to either the nipple drinkers or dish-type drinkers. The unpaired pens in each water treatment were assigned to nipple drinkers. To measure water disappearance, a water meter (Neptune T-10; Schlaumberger Canada Ltd, Mississauga, Ontario) was installed for each of the 12 pairs of pens that used a common drinker. The meters were rated to be 95% accurate at a low flow of 500 mL per minute.
Animal care and management
All procedures used in this study were approved by the University of Saskatchewan Committee on Animal Care and Supply (Protocol 960044) and conformed to standards established by the Canadian Council on Animal Care.9
Pigs were weaned at approximately 21 days of age. Each experiment consisted of all male pigs from one week’s weaned litters (approximately 56 litters), which was approximately 240 pigs. To eliminate the potentially confounding effects of litter, half of the male pigs from each litter were selected and assigned to RO-treated water and the other half to untreated water. Specific pigs within a litter were allocated to treatment to ensure that the final average weight on each treatment was similar.
Pigs remained in the nursery for 5 weeks after weaning, and were allowed ad libitum access to commercial feeds (Feed-Rite Mills, Humboldt, Saskatchewan). Pigs were offered a creep-type diet for the 10 days following weaning, and thereafter were switched to a two-phase starter system. The first diet was fed for 10 days, and the second starter for the remainder of the experiment. Animals were observed daily for signs of stress or distress. On the advice of a veterinarian, pigs exhibiting symptoms of diarrhea received either Carbadox (Pfizer Canada; Kirkland, Quebec), 3 mL daily for a maximum of 3 days, or an injection of sulfadoxine and trimethoprim (Borgal; Intervet Canada Ltd, Whitby, Ontario), 0.6 to 1.0 mL daily for a minimum of 2 days.
Experiment One: Experimental design and sample processing
Experiment One was planned as a completely randomized design with a factorial arrangement of treatments (RO-treated or untreated water and nipple drinkers or dish-type drinkers). Pigs were weighed on the day of weaning (Day 0) and on Days 18 and 35. Feed was weighed before delivery to individual pens, with remaining feed weighed and recorded twice weekly for calculation of feed intake. All diets contained 3000 mg zinc (from zinc oxide) per kg of feed.
The water meters recorded cumulative water disappearance. Spilled water was not measured; therefore, it is included in total water consumption and reported as water disappearance. Flow rates were determined weekly by allowing water to flow into a calibrated container for 1 minute. Nipples were cleaned, adjusted, or changed if the water flow was unusually low or high. Water samples for analysis were collected weekly into sterile containers after the water in each line had run for 30 seconds. Samples were collected directly from the nipple of the nipple drinkers or from the outlet nozzle in the dish-type drinkers.
Samples were submitted to the Saskatchewan Research Council (Saskatoon, Saskatchewan). Initially, samples were cultured for total coliforms and plate count and assayed for TDS, specific conductivity, pH, calcium, chlorine, bicarbonate, potassium, magnesium, sodium, sulfate, total alkalinity, total hardness, nitrate and nitrite nitrogen, total phosphorus, silver, aluminum, barium, cadmium, beryllium, cobalt, chromium, copper, iron, manganese, molybdenum, nickel, lead, soluble silicon, strontium, titanium, vanadium, zinc, and zirconium.10 Consistent results were obtained for 3 consecutive weeks; therefore, the assay profile was reduced to sulfate, lead, TDS, and pH.
To allow determination of apparent nutrient digestion, a special batch of the commercial starter diet was prepared with 0.4% chromic oxide and fed to 10 pens (five from each water treatment group, randomly chosen from pens equipped with either nipple drinkers or dish-type drinkers) from Day 14 to Day 22. Samples of feed were obtained at the time of the chromic oxide inclusion. Freshly voided feces were collected from five pigs per pen twice daily on Days 19 to 22. Morning and afternoon samples were pooled by pen and frozen. At the end of the collection period, daily samples from each pen were blended, subsampled, and lyophilized (Virtis Co, model 40-SUB; Gardiner, New York). Nutrient digestibility was calculated by comparing the ratios of nutrients to chromic oxide in the feed and feces.
Feed samples and the lyophilized fecal samples were ground through a 1-mm screen (Retsch ZM1; Brinkman Instruments Ltd, Gardiner, New York) and assayed for gross energy by bomb calorimetry (Parr Instrument Co, model 1200; Moline, Illinois), crude protein (Kjeldahl N x 6.25; Association of Official Analytical Chemists),11 acid detergent fibre,12 neutral detergent fibre,13 and chromic oxide.14 Dry matter was determined by drying at 100°C in a vacuum oven to a constant weight.12
Blood was collected by cranial vena cava venipuncture from 20 randomly selected pigs per water treatment group on Day 28 postweaning. Cell numbers and chemical constituents were determined in a commercial laboratory (Department of Veterinary Pathology, University of Saskatchewan, Saskatoon, Saskatchewan) as described by Maenz et al.5 This laboratory supplied reference means for each constituent.
Tissue samples were obtained from four pigs per water treatment group on Day 0, and from six pigs per water treatment group on Days 7 and 21 postweaning. The pigs were selected at random from each water treatment group on Day 0. On Days 7 and 21, the pigs were selected in pairs so that a littermate was chosen from each treatment group. Euthanasia was accomplished by a barbiturate overdose. Tissue samples consisted of the entire organ, or if necessary, a subsample. Approximately 50-gram samples of muscle (left hind limb), kidney, brain, and liver were removed, sealed in plastic bags, stored on ice, transported to the Saskatchewan Research Council (Saskatoon, Saskatchewan), and assayed for calcium, potassium, magnesium, sodium, sulfur, and chlorine.
Experiment Two: Experimental design
A second experiment was conducted to determine if the lack of a performance response to the poor quality water in the first experiment was due to inclusion of zinc oxide in the diet. Pigs were assigned to receive either untreated or RO-treated water, and diets were supplemented with either 0 mg or 3000 mg zinc (from zinc oxide) per kg of feed. Pigs were weighed on the day of weaning (Day 0) and on Days 21 and 33. Feed was weighed before delivery to individual pens, and remaining feed was weighed and recorded twice weekly for calculation of feed intake. Blood samples were collected from 10 pigs randomly selected from each water treatment group on Day 29.
Statistical analysis
Pen was the experimental unit for performance data, whereas data from individuals was used for the blood and tissue statistical analysis. Data from both experiments were analyzed by ANCOVA using the GLM procedure of SAS15 (SAS Institute Inc, Cary, North Carolina), with weaning weight (Day 0) employed as the covariate. The model for Experiment One included the effects of litter, water treatment, drinker type, and the interaction of drinker type and water treatment. Nutrient digestibilities, determined in Experiment One, were compared using the means and SDs. The model for Experiment Two included the effects of litter, water treatment, dietary zinc, and the interaction of dietary treatment (zinc) and water treatment. To account for the sampling day of tissue, study day and the interaction of study day and water treatment were also included in the model for these assays.
Results
Water quality and flow
Water composition is described in Table 1 using the mean, SD, and range. One sample of the RO-treated water contained higher than average concentrations of the constituents measured. These results were included in the calculation of the means and SDs. Assay results from samples collected 1 week prior to this sample and 4 days after this sample were close to the mean.
The TDS concentrations of the untreated water samples ranged from about 5% above to 3% below the concentrations recommended in the Task Force on Water Quality Guidelines1 for livestock drinking water. Sulfate concentrations of the untreated water were consistently 60 to 70% above those recommended by the Task Force.1 The total iron content (7.3 mg per mL) in the untreated water was also very high; however, there is no recommended maximum concentration for this mineral.1 Concentrations of nitrates (included nitrite), calcium, boron, copper, and fluoride were below the recommended maximum concentrations suggested.1 Total coliforms, determined in three samples from each water treatment, never exceeded 200 colony forming units (CFU) per 100 mL, below the maximum level (5000 CFU per 100 mL) suggested in the guidelines.1
The RO treatment effectively removed most of the minerals measured in the untreated water in our study, including approximately 94% of the TDS, 99% of the sulfates, 95% of the calcium and total hardness, and 94% of the iron.
Water flow averaged 1180 +/- 372 mL per minute (mean +/- SD) from the nipple drinkers and 1300 +/- 544 mL per minute from the dish-type drinkers. Because nipples were cleaned or changed if a low water flow was measured, the low flows were not consistently recorded from the same pen.
Performance
Experiment One. Two hundred and fifty-eight pigs entered the experiment, with an average age of 20.9 +/- 1.3 days (mean +/- SD) and weighing 5.9 +/- 1.0 kg. Two pigs died and 32 pigs were euthanized for the purpose of collecting tissue samples during the experiment. The 224 remaining pigs weighed 21.1 +/- 3.1 kg at completion of the 35-day experiment.
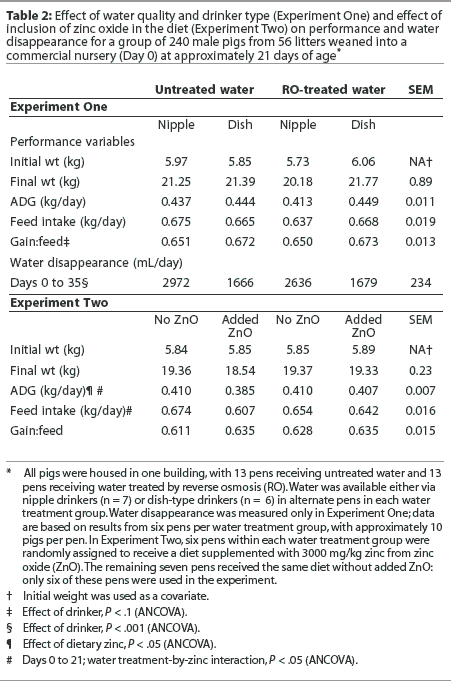
Average daily gain and ADFI were similar (P > .05) regardless of water treatment or drinker type (Table 2). The gain-to-feed ratio tended to be higher for pigs in pens equipped with dish-type drinkers than for pigs in pens with nipple drinkers (P < .1; Table 2). Water disappearance was greater in pens with nipple drinkers (P < .01; Table 2), regardless of water treatment.
Water treatment had no effect (P > .05) on apparent digestibility of dry matter (82.3% +/- 0.9, mean +/- SD), gross energy (82.3% +/- 0.8), crude protein (80.3% +/- 0.7), acid detergent fiber (25.2% +/- 4.8), or neutral detergent fiber (56.1% +/- 1.8).
Experiment Two. Pigs entered the second experiment at a mean age of 18.7 +/- 0.8 days (mean +/- SD) and a weight of 5.86 +/- 0.90 kg (n = 230). No pigs were euthanized during this study, and animal deaths were not tabulated. At the conclusion of the experimental period (33 days), the average weight was 19.2 +/- 0.92 kg. As in Experiment One, ADG, ADFI, and feed efficiency were similar regardless of water treatment group (P > .05; Table 2). Pigs offered diets without supplemental zinc oxide consumed 40 grams more feed per day (P < .05). The ADG was lower for pigs receiving a diet supplemented with zinc oxide (n = 12 pens; effect of dietary zinc; P < .05). This effect of zinc oxide on ADG was most apparent in the group receiving untreated water: a water treatment-by-zinc interaction was observed during the initial 3 weeks of the experiment (Days 0 to 21; P < .05; Table 2).
Blood constituents
Experiment One. Water treatment and drinker type had minimal effects on serum parameters. Serum bicarbonate was higher, and serum albumin and creatinine were lower, in pigs on untreated water than in pigs on RO-treated water (P < .05; Table 3). Other proteins and minerals measured in serum, or cellular constituents measured in whole blood, were similar regardless of water treatment or drinker type (P > .05). With the exceptions of calcium (2.9 +/- 0.07 mmol per L; mean +/- SEM: reference, 2.74 mmol per L), magnesium (1.32 +/- 0.04 mmol per L: reference, 0.81 mmol per L), and glucose (5.9 +/- 0.6 mmol per L; reference, 3.6 mmol per L), all values were within reference ranges (Department of Veterinary Pathology, University of Saskatchewan, Saskatoon, Saskatchewan).
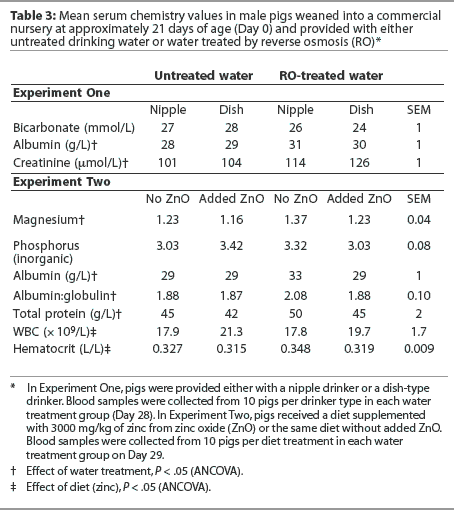
Experiment Two. Average WBC count was lower and mean hematocrit was higher in pigs consuming diets containing no supplemental zinc (P < .05; Table 3). Other serum chemistry values, including mineral and bicarbonate concentrations, were similar regardless of dietary zinc concentration (P > .05). As in Experiment One, serum albumin was higher in pigs receiving RO-treated water (P > .05) than in pigs receiving the untreated drinking water. In contrast to the results of Experiment One, the mean serum albumin:globulin ratio and total protein were higher in groups receiving the RO-treated water (P < .05).
Tissue mineral concentrations
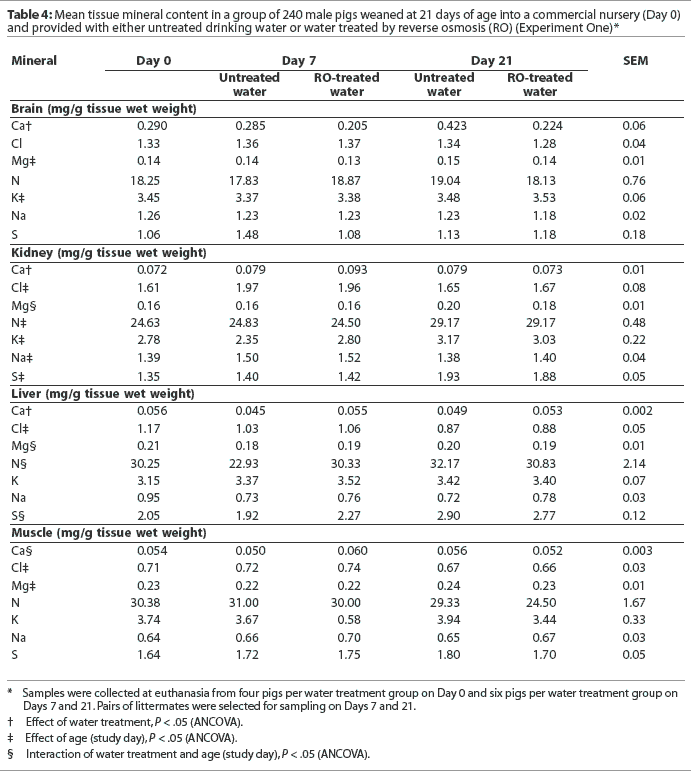
Concentrations of chlorine, magnesium, nitrogen, potassium, sodium, and sulfur in brain, liver, and muscle tissues were similar regardless of water treatment or drinker type (P > .05; Table 4). The concentration of calcium in kidney and liver tissues was higher in pigs that had received RO-treated water compared to pigs that had consumed untreated water (P < .05). However, this difference was primarily due to effects observed on Day 7. Conversely, the concentration of calcium was greater in brain tissue obtained from pigs that had consumed untreated water compared to pigs that received RO-treated water (P < .05). Concentrations of magnesium and potassium in the brain; nitrogen, potassium, and sulfur in kidney tissue; and magnesium in muscle tissue were higher in samples taken on Day 21 compared to Day 7 (P < .05). The overall concentrations of chlorine (regardless of water treatment) were higher in kidney, liver, and muscle tissue on Day 7 compared to Day 21 (P < .05). Similarly, sodium concentration in kidney tissue was greater on Day 7 compared to Day 21 (P < .05).
Discussion
A number of constituents may be contained in water at concentrations that are harmful to pigs.2 Bacterial contamination is a problem, especially in surface waters;16 however, water uncontaminated by bacteria or by other microorganisms may contain levels of dissolved solids which make it unacceptable for consumption. In the present study, poor quality water refers to water containing high concentrations of inorganic materials. The concentrations of total coliforms never exceeded 200 CFU per 100 mL, well below the standard of 5000 coliforms per 100 mL established by the Bureau of National Affairs.16 Poor quality water on swine farms is associated with an increased incidence of diarrhea and subsequent poor performance. However, the evidence is largely anecdotal and is not supported by available research data. Cognizant of possible differences between research and commercial facilities, with respect to the pig’s response to water quality, we chose to conduct this trial at a commercial farm. The experiments were conducted on a 1200-sow farrow-to-finish operation where water quality was a serious concern. Water on this farm was obtained from a deep well and was delivered to the herd after treatment by an RO unit. The concentrations of TDS and sulfates in the untreated water exceeded the Canadian guidelines for water quality established in 1987.1 The RO unit effectively reduced the concentrations of TDS. Sulfates cause nonpathogenic (osmotic) diarrhea17 and are the main contributor to poor quality water in many regions of North America.16 In a survey of 135 wells in Saskatchewan, TDS and sulfates exceeded the recommended levels in 7.4% and 25.0% of wells, respectively.3 The concentrations of these constituents in our study exceeded the mean in the Saskatchewan survey, but not the maximum values in the survey (6590 mg TDS per L and 3760 mg sulfates per L).3 The untreated water in our study had acceptable levels of other constituents, including nitrates.
There was no interaction of water treatment with drinker type in Experiment One; therefore, performance data were averaged across drinker type in Experiment Two. Consistent with the limited research on this topic, water quality in our studies was not associated with changes in pig performance, regardless of whether the criteria used were ADG, ADFI, or feed efficiency. Tremblay et al18 found that growth and ADFI were similar whether the drinking water contained 1260 mg or 75 mg sulfates per L, and Veenhuizen et al19 showed that growth and ADFI were not affected when weaned pigs received water containing up to 1800 mg sulfate per L for 28 days. Notably, the work of Tremblay et al18 was conducted both at a research facility and on a commercial farm. Moreover, when pigs received either deionized water or water obtained from farms where there was a perceived problem of water quality, pigs consuming water that was high in sulfates (> 1900 mg per L) actually gained faster than pigs receiving the deionized water.20
Water disappearance, which was measured only in Experiment One, was not affected by water quality. Concentrations of sulfates in water of 400 mg per L taste bitter to humans and are cathartic. However, adaptation to this taste in humans has been reported, and consumption of water containing high concentrations of sulfates may increase over time.21 Concentrations of 1200 mg of sodium sulfate per L of liquid feed had no effect on the intake of this feed by weaned pigs,17 and water consumption was unaffected when grower-finisher pigs were offered water containing 11.7 g of sodium or magnesium sulfate per L.22 Moreover, pigs that received drinking water containing up to 1800 mg sulfate per L or 4390 mg TDS per L (including 2650 mg sulfates per L) consumed more water than the controls receiving low-mineral water.5,19
Blood samples were submitted to a clinical pathology laboratory for analysis to identify possible subclinical health effects related to the consumption of untreated water. Water treatment had only minimal effects on the blood parameters measured. Although the concentration of serum albumin was lower for pigs receiving untreated drinking water in both experiments, the albumin:globulin ratio was lower for this treatment group in Experiment Two, and the concentration of creatinine tended to be lower in Experiment One when pigs consumed the high sulfate water. Likewise, Maenz, et al5 observed a lower serum albumin concentration when pigs consumed water with a high concentration of TDS and sulfates. Albumin is the major protein responsible for the osmolarity of the plasma,23 and an increase in serum albumin concentration, or more specifically, an increase relative to serum concentration of the other blood proteins, indicates dehydration, which would be expected if the pigs were suffering from diarrhea. However, diarrhea must be severe before changes in blood osmolarity are observed.24 For example, a high incidence of diarrhea was reported in an experiment in which pigs received saline water with high concentrations of sulfates, nitrates, and chloride; however, there was no effect on blood hematocrit or sodium or potassium concentrations.25
In Experiment One, it was shown that when the pigs consumed water with high concentrations of sulfates, there was no effect on nutrient digestibility, implying no effect on rate of intestinal passage or fecal output. This is consistent with no effect on the incidence of diarrhea after consuming water containing high concentrations of TDS or sulfates. Anderson et al22 reported that concentrations of TDS (in the form of sodium salts) up to 8000 mg per L and sulfates up to 4000 mg per L had no effect on nutrient digestibility, although there was a decrease in dietary metabolizable energy (ME) as TDS increased, possibly due to the loss of anions (ie, bicarbonate) in the urine.22 We cannot test this hypothesis directly in our experiment, since ME was not determined, and diets were adequate in energy. However, serum bicarbonate concentration was higher in pigs receiving untreated water in Experiment One. These data again refute observations reported in clinical situations of poor nutrient utilization in the presence of poor quality water.
Sulfates exist primarily as salts of magnesium or sodium. Although these were not distinguished in our water analysis, calculation of the molar proportions of sodium, magnesium, and sulfate indicates that the water might have contained similar proportions of sodium and magnesium sulfate. The magnesium ion has been implicated as the causative factor in diarrhea induced by magnesium sulfate;26 however, neither sodium nor magnesium sulfate adversely affected weanling pig performance when included in the drinking water at 3000 mg per L27 or 1800 mg per L.19
In Experiment One, all pigs received supplemental zinc from zinc oxide at a concentration of 3000 mg per kg in their diet. This level of supplemental zinc added to the diets of young pigs has been associated with improved ADG and ADFI.6,7 In Experiment Two, the effect of water quality was examined when pigs were fed diets with or without supplemental zinc oxide to determine whether the zinc was mitigating the effect of poor quality water by controlling diarrhea. In contrast to what has been observed by others,6,7,28 ADFI and ADG were lower between Day 0 and Day 21 when pigs consumed diets containing supplemental zinc. Moreover, the poorest performance was observed in groups on untreated water and consuming a diet supplemented with zinc oxide. Zinc may benefit the newly weaned pig by maintaining the protective capacity of the intestinal flora.29 When newly weaned pigs consumed water containing between 217 mg and 4390 mg TDS per L, performance was adversely affected only when the pigs also received unmedicated feed.4 Diarrhea associated with ingestion of poor quality water is thought to be of osmotic origin, due to the presence of a poorly absorbable solute in the lumen of the gut causing fluid accumulation.5 Although the effect of water quality on performance in our experiments was minimal, supplemental dietary zinc may have exacerbated rather than alleviated any effect of the poor quality water by adding to the mineral load. The albumin:globulin ratio and hematocrit were highest in pigs receiving the combination of no supplemental zinc oxide and RO-treated water, indicating that pigs receiving the untreated water were not dehydrated. It is interesting that in both experiments, blood parameters suggestive of dehydration were not consistent with changes in performance or evidence of diarrhea.
Water disappearance was highest in pigs using the nipple water dispenser, almost certainly a result of greater spillage. When nipple drinkers were compared to bowls for 4 days after weaning, water disappearance was much higher with the nipple drinkers, yet true water consumption was not affected.30 Feed efficiency in our experiment tended to be lower with the nipple drinkers, resulting from a combination of greater feed intake and reduced gain during the second half of the experiment. This is difficult to explain and contrasts with the data of Phillips and Phillips,30 who observed no effect of water-dispenser type on feed intake or performance immediately following weaning.
In conclusion, the performance of weaned pigs was not compromised by the consumption of water containing high levels of TDS and sulfates. Although the untreated water in this experiment contained a concentration of TDS 15 times greater than that of the RO-treated water, this is only slightly higher than the maximum concentration recommended by the Task Force on Water Quality.1 However, there may be situations in which other environmental, nutritional, and social stressors imposed at weaning, combined with poor quality water, may overwhelm the pigs’ immune function. These data do indicate that problems with poor growth, feed intake, or diarrhea should not be attributed to water quality problems without first investigating other contributing factors.
Implications
- Nursery pigs can tolerate water with TDS concentrations above recommended levels.
- Problems with poor growth, feed intake, or diarrhea in nursery pigs should not be attributed to water quality until other possible contributing factors have been investigated.
- High levels of dietary zinc may exacerbate problems with high concentrations of TDS in the drinking water.
Disclaimer
The use of trade names does not imply product endorsement by the authors, nor criticism of similar products not mentioned.
Acknowledgements
The authors acknowledge with gratitude the financial assistance provided by the Partnership Agreement on Water Based Economic Development administered by the Prairie Farm Rehabilitation Administration, Agriculture Development Fund, Canadian Feed Industry Association, Central Water Conditioning, SPI Marketing Group, Feed-Rite Mills Ltd, Hillcrest Farms Ltd, Kenpal Farm Products Ltd, Shamrock Feed Ltd, Masterfeeds, and Ralston Purina Canada Inc. The authors also acknowledge with gratitude the strategic funding provided to PSCI by Saskatchewan Pork, Alberta Pork, the Manitoba Pork Council, and the Saskatchewan Agriculture Development Fund. In particular, appreciation is expressed to Nadine Possberg for animal care and to the management and staff of Stomp Pork Farms for supporting this project and allowing the study to be conducted at their facility.
References
*1. Task Force on Water Quality Guidelines. Canadian Water Quality Guidelines, Inland Waters Directorate, Ottawa, Ontario. 1987.
2. National Research Council. Nutrient requirements of swine. 10th ed. Washington, DC: National Academy Press; 1998.
3. McLeese JM, Patience JF, Wolynetz MS, Christison GI. Evaluation of the quality of ground water supplies used on Saskatchewan swine farms. Can J Anim Sci. 1991;71:191-203.
4. McLeese JM, Tremblay ML, Patience JF, Christison GI. Water intake patterns in the weaning pig: effect of water quality, antibiotics and probiotics. Anim Prod. 1992;54:135-142.
5. Maenz DD, Patience JF, Wolynetz MS. The influence of the mineral level in drinking water and the thermal environment on the performance and intestinal fluid flux of newly-weaned pigs. J Anim Sci. 1994;72:300-308.
6. Hahn JD, Baker DH. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci. 1993; 71:3020-3024.
7. Carlson MS, Hill GM, Link JE. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J Anim Sci. 1999;77:1199-1207.
8. Huang SX, McFall M, Cegielski AC, Kirkwood RN. Effect of dietary zinc supplementation on Escherichia coli septicemia in weaned pigs. Swine Health Prod. 1999;7:109-111.
9. Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals. 2nd ed. Ottawa, Ontario, Canada: Canadian Council on Animal Care; 1993.
10. American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 18th ed. Washington, DC: American Public Health Association; 1992.
11. Association of Official Analytical Chemists. Official Methods of Analysis. 16th ed. Arlington, Virginia: Association of Official Analytical Chemists; 1996.
12. Association of Official Analytical Chemists. Official Methods of Analysis. 15th ed. Arlington, Virginia: Association of Official Analytical Chemists; 1990.
13. Van Soest PJ, Robertson JB, Lewis BA. Carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583-3597.
14. Fenton TW, Fenton M. An improved procedure for the determination of chromic oxide in feed and faeces. Can J Anim Sci. 1979;59:631-634.
*15. SAS User’s Guide. Version 5. Cary, North Carolina: SAS Institute Inc; 1985.
16. Thacker P. Water in swine nutrition. In: Lewis AJ, Southern LL, eds. Swine Nutrition, New York: CRC Press. 2001;381-398.
17. Gomez GG, Sandler RS, Seal ES Jr. High levels of inorganic sulfate cause diarrhea in neonatal piglets. J Nutr. 1995;125:2325-2332.
*18. Tremblay ML, Christison GI, Patience JF. The effect of sulfate in water on weanling pig performance [abstract]. Can J Anim Sci. 1989;69:1118.
19. Veenhuizen MF, Shurson GC, Kohler EM. Effect of concentration and source of sulfate on nursery pig performance and health. JAVMA. 1992;201:1203-1208.
*20. DeWit PL, Young G, Wenzell R. Friendship R, Peer D. Water quality and pig performance [abstract].Can J Anim Sci. 1987;67:1196.
21 Chien L, Robertson H, Gerrard JW. Infantile gastroenteritis due to water with high sulfate content. Can Med Assoc J. 1968;99:102-104.
22. Anderson JS, Anderson DM, Murphy JM. The effect of water quality on nutrient availability for grower/finisher pigs. Can J Anim Sci. 1994;74:141-148.
23. Harfenist EJ, Murray RK. Plasma proteins, immunoglobulins, and clotting factors. In: Murray RK, Grammer DK, Mayes PA, Rodwell VW, eds. Harper’s Biochemistry. Norwalk, Connecticut: Appleton and Lange; 1990:609.
24. Andren B, Persson S. Acid-base and electrolyte changes in 1-3 days old pigs infected with enteropathogenic Escherichia coli and in spontaneous cases of piglet diarrhoea. Acta Vet Scand. 1983;24:84-98.
25. Anderson DM, Stothers SC. Effects of saline water high in sulfates, chlorides and nitrates on the performance of young weanling pigs. J Anim Sci. 1978;47:900-907.
*26. Reichelderfer M, Pero B, Lorenzsonn V, Olsen WA. Magnesium sulfate-induced water secretion in hamster small intestine. Proc Soc Exp Biol Med. 1984;176:8-13.
27. Paterson DW, Wahlstrom RC, Libal GW, Olson OE. Effects of sulfate in water on swine reproduction and young pig performance. J Anim Sci. 1979;49:664-667.
28. Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, Lewis AJ, Miller PS, Shurson GC, Veum TL. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Anim Sci. 2001;79:934-941.
29. Katouli M, Melin L, Jensen-Waern M, Wallgren P, Mollby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol. 1999;87:564-573.
30. Phillips PA, Phillips MH. Effect of dispenser on water intake of pigs at weaning. Trans Am Soc Agric Eng. 1999;74:141-148.
* Non-refereed references