Case report |
Peer reviewed |
Idiopathic vesicular disease in a swine herd in Indiana
Sandra F. Amass, DVM, PhD, Diplomate ABVP; Jessica L. Schneider, RVT; Cheryl A. Miller, DVM; Samia A. Shawky, DVM, PhD; Gregory W. Stevenson, DVM, PhD, Diplomate ACVP; Mary E. Woodruff, MS
SFA, JLS: Department of Veterinary Clinical Sciences, Purdue University, Lafayette, Indiana. CAM: Indiana State Board of Animal Health, Indianapolis, Indiana. SAS: Foreign Animal Disease Diagnostic Laboratory, USDA, APHIS, VS, NVSL, Greenport, New York. GWS, MEW: Animal Disease Diagnostic Laboratory, Purdue University, Lafayette, Indiana. Corresponding author: Dr Sandra F. Amass, Purdue University, VCS/Lynn, 625 Harrison St, West Lafayette, IN 47907-2026; Tel: 765-494-8052; Fax: 765-496-2608; E-mail: amasss@purdue.edu
Cite as: Amass SF, Schneider JL, Miller CA, et al. Idiopathic vesicular disease in a swine herd in Indiana. J Swine Health Prod. 2004;12(4):192-196.
Also available as a PDF.
Summary
Idiopathic vesicular disease was reported in pigs of all ages during a 7-month period on an 1840-sow, three-site farrow-to-finish swine herd in Indiana. In 8- to 10-week-old growing pigs recently moved from the nursery (Site 3) to Sites 1 and 2, erosions occurred primarily on the coronary band, causing coronary separation and lameness. Some growing pigs also had lesions on the snout and in the oral cavity. A few gestating sows in Sites 1 and 2 were affected primarily with snout lesions, and some litters of piglets were febrile and had coronary band lesions that resolved by 16 days of age. Pigs in Site 3 were not affected. Clinical signs were indistinguishable from exotic vesicular diseases such as foot-and-mouth disease. A foreign animal disease investigation ruled out an exotic disease as the cause. An etiologic agent was not identified; however, porcine enterovirus was isolated from tissue samples of growing pigs. Porcine enteroviruses are ubiquitous, and the isolate was considered a contaminant in this case. Practitioners are encouraged to be vigilant observers and reporters of clinical signs consistent with exotic diseases.
Keywords: swine, idiopathic
vesicular
disease, enterovirus
Search the AASV web site
for pages with similar keywords.
Received: October
24, 2003
Accepted: February
12, 2004
Lameness, coronary band separation, vesicular lesions, or combinations of all three were observed in approximately 800 of 1000 growing pigs (8 to 10 weeks old) during a routine February herd visit on an 1840-sow farrow-to-finish swine herd in Indiana. The clinician and veterinary technologist immediately considered the exotic animal disease differentials for vesicular diseases and telephoned the State Board of Animal Health from outside the building housing the affected pigs. A Foreign Animal Disease Diagnostician (FADD) arrived at the farm within 1.5 hours.
Initial outbreak of vesicular disease
Case description
The main farm consists of two sites (Sites 1 and 2) approximately 0.8 kilometers apart, that house breeding, gestating, farrowing, and overflow finishing pigs. At 9- to 16-days of age, all pigs are weaned from these sites to an offsite nursery (Site 3) located approximately 3.2 kilometers away. Each site has separate animal caretakers. Periodically, groups of 500 to a thousand 6- to 8-week-old pigs are moved from Site 3 to Sites 1 and 2 for finishing. The affected pigs had been moved from Site 3 (curtain and mechanically ventilated wean-to-finish building with concrete slatted flooring and deep pit) to Site 2 (curtain-sided finisher with concrete slatted flooring and deep pit) 1 week before the outbreak. Pigs from the same group that had not been moved from Site 3 remained clinically healthy and free of lesions.
Affected pigs were lethargic; however, managers did not report a decrease in feed intake or an increase in mortality. A single dyspneic pig died within minutes of first observation of clinical signs. Cursory examination of this pig revealed 1-mm to 1-cm ruptured vesicles on the tongue and oral and gingival mucosa. The differential diagnoses included foot-and-mouth disease (FMD), vesicular stomatitis (VS), swine vesicular disease (SVD), vesicular exanthema of swine (VES), parvovirus, mycotoxicosis, and idiopathic vesicular disease. The lesion pattern was not consistent with a chemical burn or other contact irritation.
Lesions and sample collection
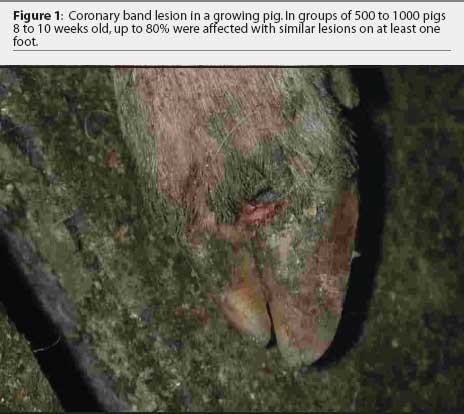
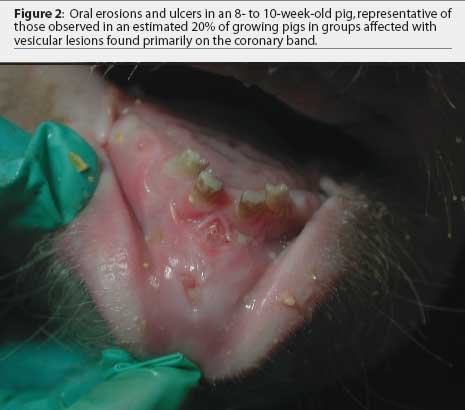
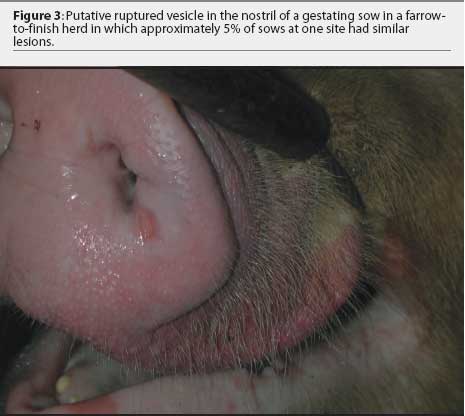
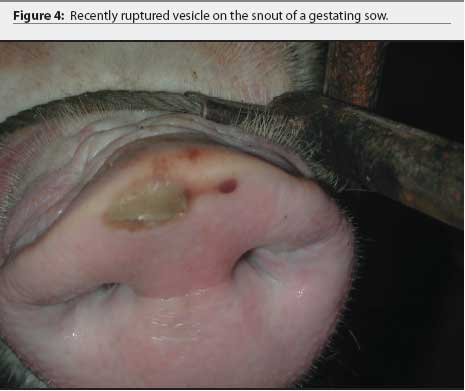
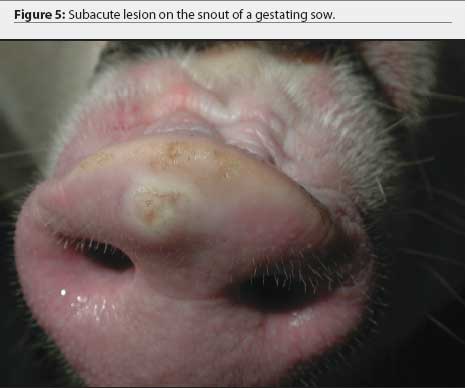
Further examination revealed that approximately 80% of growing pigs in this group had coronary band lesions on at least one foot, and approximately 20% of pigs had snout lesions consisting of well-circumscribed erosions or ruptured vesicles. The dead pig had bronchopneumonia, but no other internal lesions were noted. One growing pig with coronary band lesions was humanely euthanized. This pig had two 2- to 3-mm lesions on the tongue, interpreted as ruptured vesicles or erosions; a 4-mm lesion on the lip; and a 3-mm lesion on the oral mucosa. Ruptured vesicles were observed on the snouts or in the nares of eight gestating sows at Site 2, and fluid-filled vesicles were observed on the snouts of two other sows in the same barn. No other abnormalities were noted.
Fresh and fixed samples of tongue, snout, gingival mucosa, feet, and heart were collected from the two dead growing pigs. Blood was collected from four additional growing pigs and two sows with intact vesicles, and vesicular fluid was collected from these sows. Samples were shipped directly to Foreign Animal Disease Diagnostic Laboratory (FADDL), USDA, APHIS, VS, NVSL, Plum Island, New York.
Quarantine procedures
The farm was quarantined by the state veterinarian. The owner was notified verbally and in writing that all movement of swine from the premises was prohibited until the quarantine was released. A brief overview of vesicular diseases was discussed with farm personnel. Care of affected pigs was limited to personnel who had already been exposed, and other employees were prohibited access to affected pigs. Pig movement was minimized to the extent possible, and when not possible, was restricted to movement within affected sites. Movement was not further restricted because it was likely that the entire site was already contaminated: if an exotic disease was diagnosed, animals would be culled on the basis of site rather than building or room. Employees owning animals susceptible to vesicular diseases were instructed not to contact susceptible animals and to find designated caretakers for these animals until quarantine was released.
Diagnostic testing and results
Serum samples from infected and contact pigs were negative for antibodies to FMD (virus infection associated antigen assay) and VES (indirect immunofluorescent antibody assay). Antigen to or nucleic acid of FMD, SVD, and VS viruses were not detected in the affected feet, snout, oral mucosa, or tongue, as determined by antigen-capture ELISA, antigen complement fixation, and polymerase chain reaction (PCR) assays. The following evening, 24 hours after the samples had been submitted, the quarantine imposed by the state veterinarian was removed from the herd on the basis of the negative presumptive diagnosis, while virus isolation tests were still pending at FADDL. Later, virus isolation tests at FADDL detected a group 1 porcine enterovirus in coronary band lesions.
The farm owner consented to the herd veterinarian’s request for continued voluntary quarantine of the herd, despite removal of the state quarantine, until a diagnosis was made. Two days after the initial incident, the original veterinarian and veterinary technologist returned to the infected site with a pathologist to collect additional samples of blood, tissue, and feed. At that time, in 8-to 10-week-old growing pigs, lesions on the coronary band were most prevalent (Figure 1), and few snout or oral lesions were noted (Figure 2). Snout lesions were noted in gestating sows (Figures 3, 4, and 5). Ten growing pigs in the affected group were identified by ear tag, and serial blood samples were collected from these pigs at the time of identification and 2 and 4 weeks later. Fresh and formalinized tissue samples and blood samples were submitted to FADDL at Plum Island Animal Disease Center and to Purdue University Animal Disease Diagnostic Laboratory (PUADDL). All samples remained negative for exotic vesicular diseases. One of the ten pigs seroconverted to parvovirus. Bacteriological results were unremarkable. Enterovirus was isolated at FADDL from skin, oral mucosa, spleen, and lymph node. Enterovirus was isolated from brain at PUADDL and submitted to FADDL, where PCR was used to identify the isolate as porcine enterovirus group 1, including serotypes 1 through 7 and 11 through 13.1 Microscopic evaluation of coronary band skin revealed epithelial infarcts with acanthomatous hyperplasia and orthokeratotic hyperkeratosis. Mycotoxin screens of feed samples for aflatoxin, and deoxynivalenol, ochratoxin, zearalenone, T-2 toxin, and diacetoxyscirpenol, were negative.
Second outbreak of vesicular disease
Case description
A second group of 500 growing pigs was transported from Site 3 to Site 1 one week after the initial outbreak. One week later, 2 weeks after the initial outbreak, approximately 40% of transported pigs had coronary band lesions and three pigs had snout lesions. One pig was febrile (rectal temperature 40.7°C). Additionally, five sows at Site 1 had erosions on their snouts, interpreted as ruptured vesicles, and three litters of 7-day-old piglets at Site 1 had coronary band lesions. Piglets were afebrile and sows were otherwise asymptomatic. Previously affected Site 2 growing pigs were active. Epithelial proliferation around the coronary band was noted in 10 to 15% of pigs, and one dead pig had oral and gingival lesions 2 cm in diameter. Approximately 5% of sows at Site 2 had erosions on snouts suggestive of ruptured vesicles.
Quarantine and diagnostic test results
The herd was again quarantined by the state veterinarian. Tissue and blood samples collected from an 8-week-old pig and a 7-day-old piglet from Site 1 were split, with half of each sample submitted to FADDL to rule out an exotic disease by repeating the tests listed previously. The other half of each sample was frozen at -80°C. After FADDL results had eliminated exotic diseases from the differential, these samples were submitted to PUADDL to rule out other known pathogens and to attempt to isolate previously undetected bacteria or viruses. Results of bacterial culture, virus isolation, fluorescent antibody testing, and microscopic examination were unremarkable.
Subsequent outbreaks of vesicular disease
One month after the initial outbreak, lesions in sows and growing pigs were resolved at Sites 1 and 2. A few growing pigs had sloughed a hoof. However, approximately 150 four- to 11-day-old piglets were lethargic and lame and had coronary band lesions. Piglets of this age group continued to be affected for approximately another month, commonly in small numbers (eg, 10 to 15 pigs), but infrequently in larger numbers (eg, 150 pigs). Affected piglets were from sows of all parities, and were housed on different types of flooring (plastic or round bar). Rectal temperatures of affected piglets ranged from 39.8°C to 41.2°C, suggesting an infectious etiology. Lesions resolved by 16 days of age. Each time affected groups of piglets were observed, fresh and fixed tissues and blood were collected and submitted first to FADDL then to PUADDL to repeat the previously listed tests, but results remained unremarkable. Enterovirus has not been isolated from piglet samples. Pigs at Site 3 were never affected.
The herd was quarantined voluntarily or by the state veterinarian intermittently for approximately 7 months. The problem resolved and all quarantines have been lifted. Animals introduced to the farm have not become clinically affected. A definitive diagnosis was never made. As no records for performance of affected pigs were kept, the cost of the outbreak could not be calculated.
Discussion
The cause of the vesicular lesions in this outbreak remains unknown. New stock had not been introduced to the herd for over 1 year. There was no history of international travel by caretakers. Exotic vesicular diseases were ruled out by FADDL. Commonalities among infected pigs were not apparent. Growing pigs appeared to exhibit lesions after transport from Site 3; however, affected sows had not been transported. The stress of transport in the growing pigs might have predisposed the pigs to infection. Pigs on Sites 1 and 2 had separate caretakers. Feed mixing errors that might have caused dietary imbalances were ruled out because sows and growing pigs were fed different diets. Moreover, affected neonatal pigs were nursing sows or eating a commercial creep feed. A common corn source was used to prepare sow and pig rations. Feed samples from affected pens were negative for T-2 toxin. Although T-2 toxin is rare in North America, it has been reported to cause oral and dermal irritation in growing pigs.2 Other less common causes of vesicular disease, such as consumption of celery or parsnips, or exposure to sea lions infected with calicivirus, were ruled out due to lack of exposure.3
Parvovirus has been associated with idiopathic vesicular lesions and erosions in 1- to 4-week-old pigs.4,5 Parvovirus was not isolated from the pigs in the current case and only one of ten affected pigs tested seroconverted to parvovirus during the outbreak.
Porcine enterovirus group 1 was isolated from affected growing pigs, but not from sows or piglets described in this report. Swine vesicular disease virus, in the Enterovirus genus, causes vesicular lesions in swine; however, the pigs on this farm did not seroconvert to SVD virus, nor was SVD virus isolated from these pigs. Tentative data exists suggesting that porcine enterovirus group 3 might be associated with vesicular disease.6 In 1979, vesicular disease was reported in ten finishing pigs in a Florida herd. Exotic viruses were not isolated. Porcine enterovirus types 3 and 8 were isolated from nasal and rectal swabs of affected and unaffected pigs in the same group. However, porcine enterovirus was not considered the causal agent and the etiology remains unknown.7
Porcine enteroviruses are ubiquitous in conventional swine herds,7,8 and have been isolated from the tonsils of clinically healthy pigs at slaughter,9 manure of healthy pigs,10 and slurry from a pig farm.11 Porcine enterovirus was likely an incidental finding in this case, caused by contamination of tissues samples with manure (containing enterovirus) during collection in the swine barn. However, future animal inoculation studies with the isolate from this herd are planned as the initial step to attempt either to replicate the clinical syndrome or to definitively rule out the isolate as the etiologic agent.
Veterinarians are trained in the recognition of exotic diseases and subsequent reporting procedures. However, we do not expect to observe such diseases on routine herd visits in the United States. Thus, despite our training, we are not mentally prepared either to observe large numbers of swine with vesicular lesions, nor to deal with the emotions of herd owners and personnel, as well as our own, in the face of a potential foreign animal disease outbreak. Nevertheless, the importance of correctly handling such an outbreak cannot be overstated, due to the devastating implications of an exotic disease for agriculture and the economy of the United States.
Practitioners are encouraged to be vigilant observers and reporters of clinical signs consistent with exotic diseases. Veterinarians should periodically review classic clinical signs and aberrant presentations of exotic diseases as part of their ongoing continuing education. Moreover, emerging diseases should be considered among the differential diagnoses when unusual clinical signs present. Practically, veterinarians should maintain in their vehicles and wallets a list of emergency phone numbers, including those of their state veterinarian and nearby FADDs, for immediate accessibility (memorized phone numbers may be forgotten during emergency situations). Veterinarians should also have a practice protocol in place for cases in which the veterinarian and practice vehicle must be decontaminated or quarantined for a period of time. Finally, veterinarians should work with their clients to establish farm and personnel procedures for instances of short- and long-term quarantine or mandatory depopulation for disease eradication.
Implications
- Practitioners are encouraged to be vigilant observers and reporters of clinical signs consistent with exotic diseases.
- Differential diagnoses for vesicular disease includes foot-and-mouth disease, vesicular stomatitis, swine vesicular disease, vesicular exanthema of swine, calicivirus, parvovirus, mycotoxicosis, idiopathic vesicular disease, chemical burn, contact irritation, and consumption of celery or parsnips.
- Definitive determination of the cause of vesicular disease might be prolonged, but exotic diseases are usually ruled out within 48 hours by FADDL.
- Veterinarians should maintain in their vehicles and wallets a list of emergency phone numbers, including those of their state veterinarian and nearby FADDs, for immediate accessibility.
- Veterinarians should have a practice protocol in place for cases in which the veterinarian and practice vehicle must be decontaminated or quarantined for a period of time.
- Producers should establish farm and personnel procedures for instances of short- and long-term quarantine or mandatory depopulation for disease eradication.
References
1. Zell R, Krumbholz A, Henke A, Birch-Hirschfeld E, Stelzner A, Doherty M, Hoey E, Dauber M, Prager D, Wurm R. Detection of porcine enteroviruses by nRT-PCR: differentiation of CPE groups I-III with specific primer sets. J Virol Methods. 2000;88:205-218.
2. Osweiler GD. Mycotoxins. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. Ames, Iowa: Iowa State University Press; 1999:731-742.
3. Montgomery JF, Oliver RE, Poole WSH. A vesiculo-bullous disease in pigs resembling foot and mouth disease 1. Field cases. NZ Vet J. 1986;35:21-26.
4. Lager KM, Mengeling WL. Porcine parvovirus associated with cutaneous lesions in piglets. J Vet Diagn Invest. 1994;6:357-359.
5. Kresse JI, Taylor WD, Stewart WW, Eernisse KA. Parvovirus infection in pigs with necrotic and vesicle-like lesions. Vet Microbiol. 1985;10:525-531.
6. Knowles NJ. The association of group III porcine enteroviruses with epithelial tissue. Vet Rec. 1988;122:441-442.
7. Gibbs EPJ, Stoddard HL, Yedloutchnig RJ, Legge M. A vesicular disease of pigs in Florida of unknown etiology. Florida Vet J. 1983;12:25-27.
8. Derbyshire JB. Enterovirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. Ames,Iowa: Iowa State University Press; 1999:145-150.
9. Betts AO. Studies on enteroviruses of the pig-I. The recovery in tissue culture of two related strains of a swine polioencephalomyelitis virus from the tonsils of ‘normal’ pigs. Res Vet Sci. 1960;1:57-64.
10. Forman AJ, Pass DA, Connaughton ID. The characterization and pathogenicity of porcine enteroviruses isolated in Victoria. Aust Vet J. 1982;58:136-142.
11. Derbyshire JB, Brown EG. Isolation of animal viruses from farm livestock waste, soil and water. J Hygiene Camb. 1978;81:295-302.