Original research |
Peer reviewed |
Effect of oxytocin-supplemented semen on fertility of sows bred by intrauterine insemination
Sasha Gibson, MSc; Robert J. Tempelman, PhD; Roy N. Kirkwood, DVM, PhD
SG: Preferred Capital Management, Fairmont, Minnesota. RJT: Department of Animal Science, Michigan State University, East Lansing, Michigan. RNK: Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, Michigan. Corresponding author: Dr R.N. Kirkwood, Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, MI 48824-1314; E-mail: kirkwood@cvm.msu.edu
Cite as: Gibson S, Tempelman RJ, Kirkwood RN. Effect of oxytocin-supplemented semen on fertility of sows bred by intrauterine insemination. J Swine Health Prod. 2004;12(4):182-185.
Also available as a PDF.
Summary
Objective: To determine the efficacy of supplemental oxytocin added to the semen dose immediately prior to an intrauterine insemination.
Methods: During September 2002, mixed parity sows on two commercial farrow-to-wean units (3000 and 1500 PIC sows) were inseminated at their first estrus after weaning (weaned sows; n = 442) or at a repeat estrus following a previous failure to successfully conceive (repeat sows; n = 100), using either a cervical (199 sows) or intra-uterine AI catheter. For sows inseminated using the intrauterine catheter, the semen did (172 sows), or did not (171 sows), contain 5 IU oxytocin. Semen age at insemination varied from 2 to 8 days.
Results: Farrowing rates and subsequent litter sizes were not affected by treatment. However, an interaction between treatment and the number of inseminations during estrus indicated a higher farrowing rate (P = .02) if oxytocin was included in the semen for sows bred only once. While semen age had no effect on litter size, semen age > 4 days was associated with a reduced farrowing rate (P < .05). Farrowing rates were lower (P < .05) for repeat sows (63.7%) than for weaned sows (84.9%), but litter size was not affected.
Implications: The use of intrauterine insemination does not improve fertility of sows. Inclusion of oxytocin in extended semen may benefit sow fertility when breeding management may otherwise result in a smaller sperm cell reservoir in the oviduct.
Keywords: swine, sows,
intrauterine insemination, oxytocin, fertility
Search the AASV web site
for pages with similar keywords.
Received: September
12, 2003
Accepted: December
16, 2003
The use of artificial insemination (AI) of swine has grown since 1990, when < 5% of sows were being bred by AI; approximately 60% of sows are now being bred by AI.1 However, in commercial practice, the reproductive performance of sows bred by AI is often lower than that achievable with natural breeding.2 The ultimate objective of the breeding protocol is to have a sufficient number of sperm cells in the oviductal sperm cell reservoir in the 24-hour period preceding ovulation,3 and any action that reduces this reservoir may compromise fertility. With AI, such a reduction in the sperm cell reservoir may result from poor timing of semen deposition relative to time of ovulation,4 or inadequate stimulation of the sow during and after insemination resulting in reduced myometrial contractions5 and a poorer sperm cell transport to the oviduct. Also, excess semen reflux (backflow) during insemination may reduce fertility,6 presumably by reducing the potential size of the sperm cell reservoir.
The boar ejaculate contains high levels of estrogens,2 and improvements in sow fertility have been noted following insemination of semen doses containing supplemental estrogen.7,8 Estrogen stimulates myometrial contractions9 via an estrogen-induced local release of prostaglandin F2µ (PGF).2,9 Additionally, the presence of a boar during estrus stimulated the endogenous release of oxytocin and enhanced uterine contractions.5 The endogenously stimulated uterine contractions are coordinated, with contraction waves being in the tubal direction,10 which presumably enhances the sperm cell reservoir.
Previous studies have shown improved sow fertility when PGF or oxytocin has been added to the semen dose,11-13 but responses have been variable.14 The reason for the variable response is unknown, but possibly reflects pre-existing farm performance and breeding management. Further, we speculate that since semen backflow occurs in most sows,6 and backflow may increase with inclusion of uterotonic hormones,15 the backflow may effectively limit the amount of active hormone available to stimulate the uterus. Therefore, if backflow were reduced, a more consistent response to oxytocin may ensue. Although it has not been critically assessed, anecdotal evidence suggests that semen backflow is less with intrauterine insemination. The reason for this effect is unknown, but may involve a smaller pool of semen immediately anterior to the catheter tip. The present study was undertaken to determine the efficacy of supplemental oxytocin added to the semen dose immediately prior to an intrauterine insemination.
Materials and methods
This study employed 542 mixed parity sows housed on two commercial farrow-to-wean swine operations during September 2002. The farms housed either 3000 PIC Cam-borough sows or 1500 PIC Camborough line 14 and line 15 sows. The target lactation length of each herd was 19 days (range 14 to 21 days), and sows were weaned into individual gestation stalls. Estrus detection of weaned sows involved boar contact in front of the stalls once daily starting on day 3 after weaning. Additionally, sows bred in previous weeks were exposed to the boar to facilitate detection of estrus in nonpregnant sows (repeat sows). At detection of the first or repeat estrus, sows were inseminated with 2.5 x 109 sperm cells in 75 mL Enduragard extender (Minitube, Verona, Wisconsin). Insemination was repeated 24 hours later if sows still exhibited estrous behavior. Third estrus inseminations were not allowed. Age of semen at insemination was 2 to 8 days.
At the time of AI, sows were assigned to AI using either a sponge-tip cervical catheter (Golden Pig; IMV International Corp, Minneapolis, Minnesota; control, n =199); an intrauterine AI catheter (Deep Golden Pig; IMV; DGP, n =172); or the intrauterine catheter but with 5 IU oxytocin (Prolabs Ltd, St Joseph, Missouri) added to the semen dose immediately prior to insemination (DGP+OT; n =171).
The dose of oxytocin included in the semen was similar to that shown to be effective by others.12 Assignment to treatment was serial, ie, first sow, treatment 1; second sow, treatment 2; third sow, treatment 3; fourth sow, treatment 1. A total of 442 sows were inseminated at their first estrus after weaning, and 100 sows were inseminated at a first repeat estrus. Sires were equally represented among treatments. Data recorded were service outcome (farrowed or returned) and subsequent total born litter size.
Prior to data analysis, parities were classified as 1, 2, or > 2; the wean-to-estrus intervals as < 6 days, 6 to 14 days, or > 14 days; and semen age as 2 to 4 days or 5 to 8 days. The semen age at the last insemination was used for data analysis because it was reasoned that this insemination was likely closest to the time of ovulation. Data were analyzed using SAS statistical software (SAS Institute, Cary, North Carolina). A multifactorial logistic model based on SAS PROC GENMOD was used to analyze farrowing rates and yielded a controlled farrowing rate, calculated after including all the covariates in the model. Terms included in the model were farm, treatment, number of inseminations during estrus (1 or 2), postweaning estrus of insemination (1 or 2), parity, semen age, and all first order interactions. Data for total born litter size were subjected to analysis of variance using SAS PROC MIXED with the model including the same terms as above. The least squares means option in the SAS procedures was used to provide adjusted means for both analyses. To analyze the effects of wean-to-estrus interval, the above models were re-run, but limited to weaned sows (estrus of insemination = 1).
Results
There was no overall effect of treatment on either controlled farrowing rate (hereafter referred to as farrowing rate) or subsequent litter size, but various interactions were detected. There was an interaction between treatment and number of inseminations during estrus. Specifically, when sows received two inseminations, farrowing rates were not different, but when sows received only one insemination, the addition of oxytocin to the semen was associated with an increased farrowing rate (Table 1). The number of inseminations was not recorded for six sows.
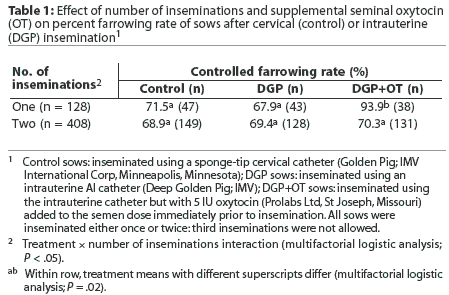
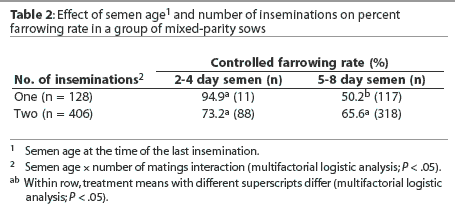
The use of semen > 4 days old was associated with a lower farrowing rate (58.1%) than when semen used was <= 4 days old (87.7%; P <.05). However, this result should be treated with caution since, as shown in Table 2, most sows received the older semen. An interaction between semen age and number of matings was detected. Specifi-cally, semen age had no effect on farrowing rates when sows were inseminated twice, but older semen was associated with a reduced farrowing rate following single inseminations (Table 2). Semen age was not recorded for two sows. Farrowing rates were lower for repeat sows (63.7%) than for weaned sows (84.9%, P < .05).
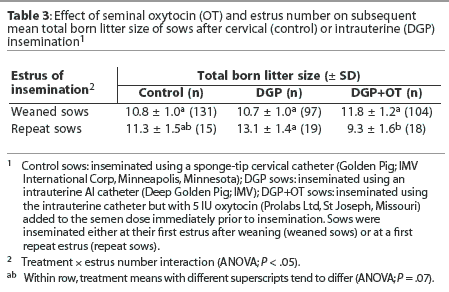
Subsequent litter size was not influenced overall by treatment (P > .05) or estrus number (wean or repeat; P > .05), but a treatment-by-estrus number interaction was detected (Table 3). Litter sizes were similar for weaned sows, but for intrauterine-inseminated repeat sows, litter size tended to be lower for DGP+OT sows, with controls being intermediate (Table 3).
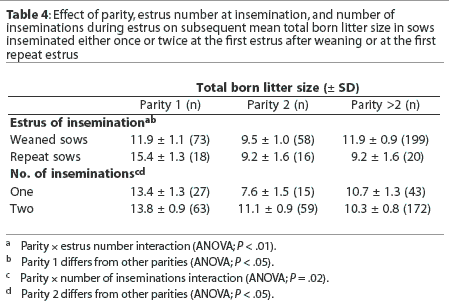
There was an overall effect of parity (P < .01) and interactions between parity and estrus number and between parity and number of inseminations during estrus (Table 4). Specifically, litter size was greater for parity 1 repeat sows than for parity 1 weaned sows (Table 4). Also, litter size was lower in parity 2 sows receiving a single insemination (Table 4).
Analysis of the effect of wean-to-estrus interval on farrowing rates and subsequent litter sizes was limited to weaned sows. Within the wean-to-estrus categories of < 6 days, 6 to 14 days, and > 14 days, the number (and percent) of sows receiving a single insemination were 57 (23.3%), 39 (28.3%), and 12 (22.6%), respectively. There was no effect of wean-to-estrus interval on farrowing rate. There was no overall treatment effect on litter size for sows returning to estrus < 6 days after weaning. However, for wean-to-estrus intervals of 6 to 14 days, litter sizes were different (P < .05) between control sows (9.6) and DGP sows (11.8), with DGP+OT sows being intermediate (10.7). When wean-to-estrus intervals were > 14 days, litter size was maintained in DGP+OT sows (13.0), but was lower (P < .05) in control sows (6.1) and DGP sows (6.0).
Discussion
The present study demonstrated no overall effect of either the site of semen deposition or the inclusion of oxytocin in the semen on farrowing rates or subsequent litter size. This supports the suggestion that no positive or negative effects will accrue from use of intrauterine insemination. It is worth emphasizing that the perceived benefit from the use of intrauterine insemination is limited to situations of insemination of low sperm cell numbers, which did not occur in the present study. Although no main effects of site of semen deposition or supplemental oxytocin were noted, various interesting interactions were evident.
The interaction between treatment and number of inseminations on farrowing rate, ie, higher farrowing rates in single-inseminated DGP+OT sows, may involve the ability to establish an effective sperm cell reservoir. If sows receive only a single insemination, there is the potential for a smaller sperm cell reservoir within the oviduct. In this event, fertility may be compromised unless the timing of insemination relative to ovulation is optimal. The higher farrowing rates for DGP+OT sows receiving a single insemination may be due to oxytocin-induced increased uterine contractions improving sperm cell transport and so allowing the establishment of an adequate oviductal sperm cell reservoir. This is supported by the lack of effect of oxytocin in sows receiving two inseminations, where a smaller sperm cell reservoir is less likely. This observation also indirectly argues against an oxytocin-induced increase in semen backflow following intrauterine semen deposition. However, since we did not assess amounts of semen backflow, this suggestion is speculative.
It is interesting that farrowing rates were not increased following two inseminations, compared to sows receiving single inseminations. This may be a consequence of single-inseminated sows having shorter estrous periods and, therefore, being inseminated relatively close to the time of ovulation. Also, the farm protocol of limiting sows to only two inseminations may have adversely affected the fertility of this group. It has been suggested that about 20% of sows will have prolonged estrous periods requiring a third insemination.16 There is anecdotal field evidence that the breeding of these sows would result in a 5% increase in the herd farrowing rate. The relatively high proportion of sows receiving single inseminations across all wean-to-estrus categories and the relatively low farrowing rates across treatments further indicate that breeding management could be improved.
The present data appear not to support the use of a long-term semen extender beyond 4 days. However, these data should be treated with caution, given the unbalanced nature of the data. When sows received a single insemination, older semen was associated with a lower farrowing rate. It is possible that the older semen doses had fewer viable sperm cells, and this resulted in a smaller oviductal sperm cell reservoir. This is supported by there being no effect when sows received two inseminations. However, if there had been fewer viable sperm cells, a semen age x treatment interaction might be expected, but none was evident.
For the farms in this study, the largest litters were obtained from the insemination of primiparous sows. Although the estrus at insemination and number of inseminations during estrus did not affect the overall subsequent litter size, there were interactions between parity and number of inseminations and between parity and estrus number. Considering the number of inseminations, when sows received two inseminations, there was a decrease in litter size in the multiparous sows, for which we have no explanation. When sows received a single insemination, there was a marked decrease in litter size for second parity sows, with a recovery in older sows. We have no explanation for the apparent "sensitivity" of second parity sows to a single insemination. When considered by estrus number, weaned sows showed the classic "second parity dip," with recovery thereafter. The parity 1 repeat sows had the largest litter sizes, possibly reflecting the known effect of "skip-a-heat" breeding in this parity.17 The repeat sows also had smaller litters in their second parity, but there was no recovery thereafter. Therefore, although there was no overall detrimental effect of estrus number on litter size, these data suggest that, except for primiparous sows, fertility is lower for repeat sows than for those conceiving at the first estrus after weaning.
The control sows demonstrated the expected decrease in litter size when insemination occurred at 6 to 14 days after weaning. However, there was no recovery for inseminations occurring at > 14 days. In contrast, there was a slight improvement in litter size of DGP sows bred between 6 and 14 days, suggesting that intrauterine insemination may be beneficial to otherwise relatively less fertile sows. Interestingly, even DGP sows had smaller litters when the wean-to-estrus interval was > 14 days. When oxytocin was included in the semen dose, litter size was maintained at the level seen with sows bred at < 6 days. Considering the above, it is possible that part of the reduction in fertility associated with breeding at > 6 days after weaning involves a failure to establish an adequate oviductal sperm cell reservoir.
The data presented demonstrated the expected reduced farrowing rate following insemination of repeat-breeder sows. The reason for this effect is not known, but in the absence of evidence to the contrary, it has been assumed to reflect a subpopulation of sows with lower fertility. However, this assumption is not supported by the data for litter sizes. On the basis of these data, it is reasonable to suggest that ovulation rate is not different in repeat-breeding sows. Similarly, in those repeat-breeding sows that maintain their pregnancy, embryo-fetal mortality is unaffected. We have no explanation for the etiology of the lower farrowing rate of this group of sows, but in the absence of an effect on litter size, it does appear to be an all-or-nothing effect.
Implications
- The use of intrauterine insemination does not improve fertility of sows.
- Inclusion of oxytocin in extended semen may benefit sow fertility when breeding management may otherwise result in a smaller sperm cell reservoir in the oviduct.
References
1. Singleton WL. State of the art in artificial insemination of pigs in the United States. Theriogenology. 2001;56:1305-1310.
2. Claus R. Physiological role of seminal components in the reproductive tract of the female pig. J Reprod Fertil. 1990;40(suppl):117-131.
3. Soede NM, Wetzels CCH, Zondag W, de Koning MAI, Kemp B. Effects of time of insemination relative to ovulation, as determined by ultrasonography, on fertilization rate and accessory sperm count in sows. J Reprod Fertil. 1995;104:99-106.
4. Kemp B, Soede NM. Relationship of weaning-to-estrus interval to timing of ovulation and fertilization in sows. J Anim Sci. 1996;74:944-949.
5. Langendijk P, Bouwman EG, Schams D, Soede NM, Kemp B. Effects of different sexual stimuli on oxytocin release, uterine activity and receptive behavior in estrous sows. Theriogenology. 2003;59:849-861.
6. Steverink DWB, Soede NM, Bouwman EG, Kemp B. Semen backflow after insemination and its effect on fertilisation in sows. Anim Reprod Sci. 1998;54:109-119.
7. Claus R, Moshammer T, Aumuller R, Weiler U. Replenishment of AI-doses with oestrogens in physiological amounts: effect on sow prolificacy in a field trial. Zentralbl Veterinarmed A. 1989;36:797-800.
8. Kirkwood RN, Thacker PA. The influence of adding estradiol to semen on reproductive performance of sows. Can J Anim Sci. 1991;71:589-591.
9. Willenburg KL, Knox RV, Kirkwood RN. Effect of estrogen formulation and its site of deposition on serum PGFM concentrations, uterine contractility, and time of ovulation in artificially inseminated weaned sows. Anim Reprod Sci. 2004;80:147-156.
10. Claus R, Hoang-Vu C, Ellendorff F, Meyer HD, Schopper D, Weiler U. Seminal oestrogens of the boar: origin and functions in the sow. J Steroid Biochem. 1987;27:331-335.
11. Huhn U, Fritzsch M, Dahms R. Control of fertility outcome in artificially inseminated gilts and old sows. 2: Addition of oxytocin to boar semen. Its effect on length of insemination, pregnancy rate and litter size. Arch Exp Veterinarmed. 1977;31:561.
12. Pena FJ, Dominguez JC, Alegre B. Effect of addition of oxytocin to seminal doses on seasonality of pig reproduction. Vet Rec. 1998;143:54.
13. Horvat G, Bilkei G. Exogenous prostaglandin F2 alpha at time of ovulation improves reproductive efficiency in repeat breeder sows. Theriogenology. 2003;59:1479-1484.
14. Flowers WL, Esbenshade KL. Optimizing management of natural and artificial matings in swine. J Reprod Fertil. 1994;48(suppl):217-228.
15. Langendijk P, Bouwman EG, Kidson A, Kirkwood RN, Soede NM, Kemp B. Functions of myometrial activity in sperm transport through the genital tract and fertilization in sows. Reproduction. 2002;123:683-690.
*16. Kirkwood RN. When to breed and when not to breed. Swine Health Prod. 2000;8:191.
17. Clowes EJ, Aherne FX, Foxcroft GR. Effect of delayed breeding on the endocrinology and fecundity of sows. J Anim Sci. 1994;72:283-291.
* Non-refereed reference.