Original research |
Peer reviewed |
Evaluation of tylosin tartrate in drinking water for treatment of porcine proliferative enteropathy (ileitis)
M. A. Paradis, DMV; G. E. Pauling, DVM, MSc; J. Brennan, PhD; N. L. Winkelman, DVM; R. N. Bagg, DVM; C. P. Dick, DVM, MSc; J. Wilson, DVM, DVSc, PhD
MAP, GEP, RNB, CPD: Elanco Animal Health, Division Eli Lilly Canada Inc, 150 Research Lane, #120, Guelph, Ontario, Canada N1G 4T2. JB: Maple Leaf Foods Agresearch, 150 Research Lane, Guelph, Ontario, Canada N1G 4T2. NLW: Swine Services Unlimited Inc, 10 E 6th Street, Morris, MN 56267. JW: Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada N1G 2W1. Corresponding author: Dr M. A. Paradis, Elanco Animal Health, Division Eli Lilly Canada Inc, 1050 Seminaire Blvd N, Suite 200, St-Jean-Sur-Richelieu, Québec, Canada J3A 1S7; Tel: 450-349-9539; Fax: 450-349-9541; E-mail: PARADIS_MARIE_ANNE@Lilly.com
Cite as: Paradis MA, Pauling GE, Brennan J, et al. Evaluation of tylosin tartrate in drinking water for treatment of porcine proliferative enteropathy (ileitis). J Swine Health Prod. 2004;12(4):176-181.
Also available as a PDF.
Summary
Objectives: To evaluate the effectiveness of tylosin tartrate administered via drinking water to pigs orally challenged with Lawsonia intracellularis.
Materials and methods: The study consisted of three treatments (Nonchallenged- unmedicated, Challenged-unmedicated, and Challenged-medicated), with six pens of six pigs per treatment (N = 108 pigs). Pigs 28 days old were challenged orally on Days 0 and 1 with L intracellularis. Drinking water medicated with tylosin tartrate (Tylan Soluble Powder; Elanco Animal Health, Indianapolis, Indiana) was provided to medicated pens Days 10 to 17. Pigs were weighed daily and assigned clinical scores, and feed consumption was measured. At necropsy (Days 17 and 18), gross lesions consistent with porcine proliferative enteropathy (PPE) were recorded, and tissues were collected for histopathologic examination and testing by polymerase chain reaction (PCR).
Results: Among challenged pigs, those medicated with tylosin had lower mean fecal consistency scores (P < .05), fewer pigs per pen with PPE-associated lesions (P < .05), less PCR-positive ileal tissue (P < .05), and better growth parameters (P < .05), compared to unmedicated pigs. Among unmedicated pigs, those challenged with L intracellularis had higher mean fecal consistency scores (P < .05), more pigs per pen with PPE-associated lesions (P < .05), more pigs PCR-positive for L intracellularis (P < .05) and poorer growth parameters (P < .05), compared to nonchallenged pigs.
Implications: This study provides evidence that tylosin tartrate administered in drinking water is effective in reducing clinical signs, lesions, and suppression of growth performance associated with PPE in swine challenged with L intracellularis.
Keywords: swine, porcine
proliferative
enteropathy, tylosin tartrate, growth
Search the AASV web site
for pages with similar keywords.
Received: March
31, 2003
Accepted: May
22, 2003
Porcine proliferative enteropathy (PPE) is an important enteric dis- ease of swine, causing significant morbidity and mortality in swine herds throughout the world. The name "PPE" describes a group of diseases associated with infection of enterocytes by the obligate intracellular bacterium, Lawsonia intracellularis, and characterized by hyperplasia of crypt enterocytes.1,2
The main clinical manifestations of PPE include the acute form, porcine hemorrhagic enteropathy (PHE), and the chronic form, porcine intestinal adenomatosis (PIA). Porcine hemorrhagic enteropathy usually occurs in pigs 4 to 12 months of age, particularly replacement breeding stock.2 Clinical signs include loose, watery stools with or without blood, gauntness, and high morbidity and mortality. Stressful events, such as mixing or sorting, shipping, overcrowding, and weaning, often precede an outbreak of PHE. Porcine intestinal adenomatosis commonly occurs in postweaned pigs 6 to 20 weeks of age. Failure to gain weight is the usual manifestation; other signs may include mild to moderate diarrhea, dullness, and apathy.2
Control of PPE is dependent in large part on the prudent administration of effective antimicrobial agents.2,3 The macrolide antibiotic tylosin is effective in the control of PPE when administered in feed or by intramuscular injection.4-7 The objective of this study was to evaluate the efficacy of tylosin tartrate administered in drinking water for treatment of experimentally induced PPE, using a mucosal challenge model.
Materials and methods
Animals and housing
One hundred and eight clinically normal pigs, obtained from a commercial farrowing unit at 14 +/- 3 days old, were used in this study. The source herd was negative for swine dysentery, transmissible gastroenteritis, and Actinobacillus pleuropneumoniae, as determined by historical records. Pigs received injectable iron prior to weaning, but no vaccines were administered.
Pigs were penned in groups of six in an isolated nursery that had been thoroughly cleaned and disinfected prior to their introduction. Each pen measured 0.9 m x 1.2 m and had fully slatted floors. Lighting program, heating, ventilation, and other management procedures were typical of modern intensive swine farms in Canada. Water was provided ad libitum by one nipple drinker per pen during the nonmedication period. During the medication period, water was supplied for all treatment groups in identical covered, calibrated reservoirs suspended above the pens. This enabled water disappearance per pen to be measured and the tylosin tartrate to be administered where appropriate. Feed was provided ad libitum in one feeder (121.92 cm of feeding space per pen). Pigs were fed a commercial, unmedicated complex diet from Day -13 to Day -2 (phase 1) and a commercial diet from Day -1 until trial termination on Day 17 (phase 2). Both diets were analyzed for crude protein, calcium, phosphorus, and sodium prior to feeding (Shur-Gain Laboratory, St Marys, Ontario, Canada). The phase 2 diet was also analyzed for tylosin activity (Elanco Animal Health Customer Services Laboratory, Corporate Center, Indianapolis, Indiana) and bacitracin activity (AT Laboratories, Memphis, Tennessee).
Study design
Pigs were blocked on initial body weight, and treatments were randomly allocated at the pen level in a randomized complete block design. The study consisted of three treatments, with six pens of six pigs per treatment group, for a total of 108 pigs. Treatment groups were Nonchallenged-unmedicated, Challenged-unmedicated, and Challenged-medicated (tylosin tartrate in the drinking water). Pens of challenged groups were separated from pens of nonchallenged controls by at least one empty pen. After allocation to pens, one animal per pen was tested serologically for L intracellularis using an immunoperoxidase monolayer assay.8 After a 13-day acclimation period (Day -13 to Day -1), L intracellularis was administered on Day 0 and Day 1 to Challenged-unmedicated and Challenged-medicated groups. Medicated water containing tylosin tartrate was administered to Challenged-medicated pigs on Days 10 through 17. All pigs were sacrificed by block, commencing on Day 17 and ending on the morning of Day 18.
Preparation of challenge inoculum
The challenge inoculum was prepared from mucosa harvested from the intestines of pigs having gross lesions consistent with PPE. Mucosa was diluted with sucrose phosphate glutamine (SPG) buffer and blended to obtain a uniform homogenized suspension.9 Samples of the homogenate were culture-negative for Salmonella serovars and Brachyspira hyodysenteriae, and were also negative by slide agglutination for Escherichia coli serotypes commonly associated with diarrhea in pigs in the herd at the time of the study.
Administration of challenge inoculum
The challenge inoculum contained 3 x 106 L intracellularis per mL on Day 0 and 1 x 106 L intracellularis per mL on Day 1, suspended in SPG buffer. The inoculum was administered via stomach tube to each challenged pig on Day 0 (32 mL) and on Day 1 (25 mL) for a total dose of 1.2 x 108 L intracellularis per pig. Nonchallenged control pigs received a dose of 32 mL SPG on Day 0 and 25 mL of SPG on Day 1. In order to minimize cross-infection of nonchallenged control pigs, SPG was administered to controls before the inoculum was administered to challenged pigs. In addition, in the areas where the pigs were inoculated, floors and pen dividers were washed and disinfected immediately following administration of inoculum, and dedicated boots, coveralls, and a footbath were used for each entry to the challenged pens.
Administration of tylosin tartrate
The test article, Tylan Soluble Powder (Elanco Animal Health, Indianapolis, Indiana), was formulated with 100% tylosin tartrate. Medicated drinking water was mixed at a rate of 1 gram of tylosin tartrate per 12 liters of water (83.3 mg per liter), with a target dose of 8 mg per kg of tylosin tartrate per day. Medicated water was mixed and issued to reservoirs above each medicated pen daily, beginning when a fecal consistency score > 1 (Table 1) was observed in more than seven pigs (10%) among challenged groups, and ending on Day 17. At the time of writing, tylosin tartrate is not registered for the control of PPE in swine.
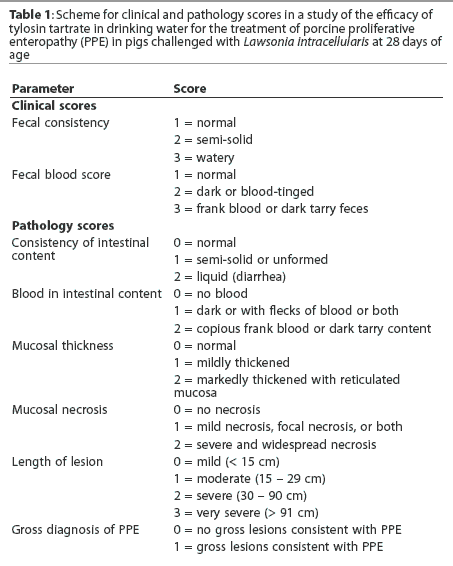
Clinical scoring
Beginning on Day 10, individual pigs were scored daily for fecal consistency and fecal blood (Table 1), using a range of 1 (normal) to 3 (marked change). Individuals responsible for clinical scoring were blind to treatment.
Production parameters
Body weights were measured on a pen basis on Day -13, Day -1, Day 14, and prior to euthanasia. Pigs that died were also weighed. Remaining feed was weighed on Day -1, Day 14, and the day of euthanasia. Water disappearance per pen for the medicated pigs was recorded daily during the medication period. Animal days were calculated for each pig from Day 14 to the day on which each animal was euthanized. Daily gain per pig was calculated by dividing weight gain by pig days. Daily feed per pig was total feed consumed per pen divided by total pig days per pen. All experimental procedures were conducted in accordance with the guidelines of the Canadian Council on Animal Care (1993).10
Necropsy procedures
All dead or sacrificed pigs received a routine postmortem examination. The ileum, cecum, and colon were opened and scored for consistency of content, blood in content, mucosal thickness, mucosal necrosis, gross diagnosis of PPE, and length of affected gut (Table 1). The following sections of the intestinal tract (approximately 3 cm in length) were collected and placed in 10% buffered formalin for histopathologic examination: ileum, both 10 and 50 cm proximal to the ileocecocolic junction; jejunum; apex of the cecum; proximal part of the ascending colon; and apex of the spiral colon. When gross evidence of PPE was present, additional sections containing gross lesions consistent with PPE were collected. All tissues were stained with hematoxylin and eosin and were scored for mucosal epithelial proliferation, proprial inflammation, mucosal necrosis, and crypt abscessation on a scale of 0 to 3: 0 = none, 1 = mild, 2 = moderate, 3 = marked. Tissues were also stained by the Warthin-Starry technique to identify intraepithelial bacteria and were similarly scored on a scale of 0 to 3. Sections of ileum taken 10 cm proximal to the ileocecocolic junction were tested for L intracellularis by polymerase chain reaction (PCR) (Dr Connie Gebhart, University of Minnesota).
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) using the pen as the experimental unit. For binomial parameters, proportions were subjected to an arcsine transformation prior to analysis. For continuous parameters, means were compared using a Student Newman Keul’s multiple range test.11
Results
Body weight, feed intake, average daily gain, and ratio of feed to gain were similar for the three treatment groups during the acclimation period (P > .05). Mean daily water disappearance per pig was 1.66 litres during the 7-day medication period and includes a water wastage factor of 40%, estimated from water turnover rates for pigs similar in body weight.12 Medicated water contained 83.3 mg tylosin per liter; therefore, apparent intake of tylosin was 83.3 mg per pig per day (8.7 mg per kg per day). All feeds were within expected ranges for nutrient content and contained no detectable tylosin or bacitracin activity.
Among challenged pigs, those receiving tylosin had lower mean fecal consistency scores on Day 12 and from Days 14 to 17 (Figure 1). Higher fecal consistency scores were observed on Days 10 to 12 and Days 14 to 17 for Challenged-unmedicated pigs compared to Nonchallenged-unmedicated pigs (Figure 1). No fecal blood was observed during this study.
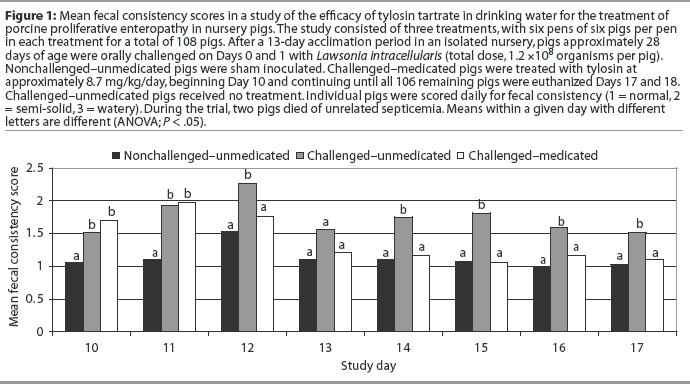
Fewer Challenged-medicated pigs per pen had gross lesions consistent with PPE, mean ileal lesion length scores were lower, and proportions positive for L intracellularis by PCR were lower, compared to Challenged-unmedicated pigs (Table 2). More Challenged-unmedicated pigs per pen had gross lesions consistent with PPE, mean ileal lesion length scores were higher, and proportions positive for L intracellularis by PCR were higher, compared to Nonchallenged-unmedicated pigs (Table 2). All nonchal-lenged pigs were negative for L intracellularis by PCR. No PPE mortality was observed during this study, although two pigs died from unrelated septicemia (one Challenged-medicated pig and one Challenged-unmedicated pig). Mortality data were not analyzed due to the small number of animals involved.
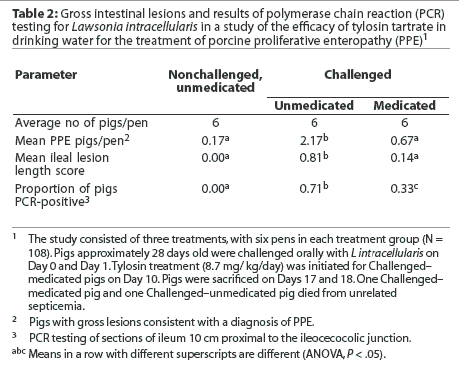
A smaller proportion of Challenged-medicated pigs had histologic lesions typical of L intracellularis infection, and mean Warthin-Starry scores were lower for all tissues examined, compared to Challenged-unmedicated pigs (Table 3). A greater proportion of Challenged-unmedicated pigs had histologic changes typical of L intracellularis infection and mean Warthin-Starry scores were higher for all tissues examined, compared to Nonchallenged-unmedicated pigs. No nonchallenged pigs had histologic lesions consistent with L intracellularis infection.
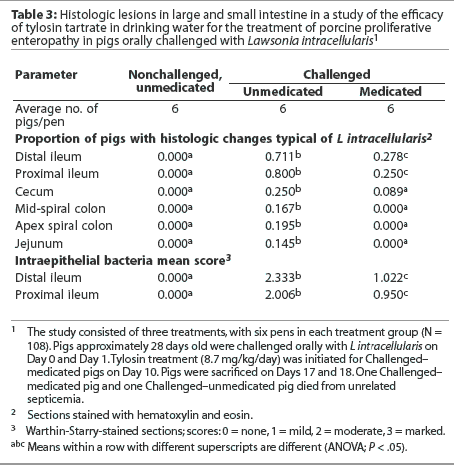
Challenged-medicated pigs had greater body weights at trial termination, greater feed intakes Day 14 to trial termination, and greater average daily gains Day 14 to trial termination and Day -1 to trial termination, compared to Challenged-unmed-icated pigs (Table 4). Challenged-medicated pigs also had lower ratios of feed to gain Day -1 to Day 14, Day 14 to trial termination, and Day -1 to trial termination, compared to Challenged-unmedicated pigs (Table 4).
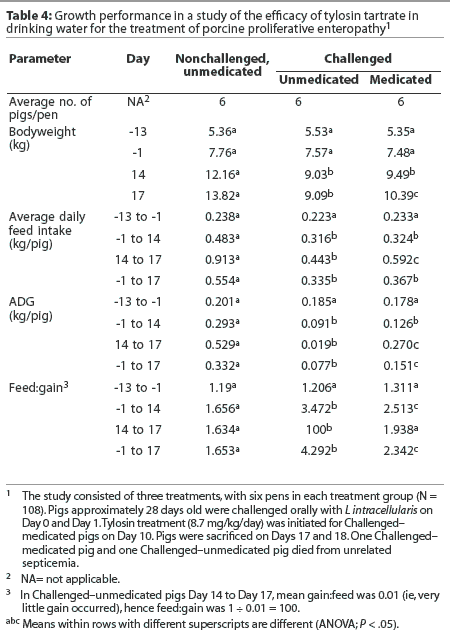
Challenged-unmedicated pigs had lower body weights Day 14 and at trial termination; lower feed intakes Day -1 to Day 14, Day 14 to trial termination, and Day -1 to trial termination; lower average daily gains Day -1 to Day 14, Day 14 to trial termination, and Day -1 to trial termination; and greater ratios of feed to gain Day -1 to Day 14, Day 14 to trial termination, and Day -1 to trial termination, compared to Nonchal-lenged-unmedicated pigs (Table 4).
Discussion
The results of this investigation demonstrate the in vivo effectiveness of tylosin tartrate administered via the drinking water in reducing the incidence and impact of PPE among pigs experimentally infected with L intracellularis. Administration of tylosin for 7 days was associated with significantly fewer gross and histologic lesions of PPE, lower fecal consistency scores, fewer pigs with PCR-positive ileal sections, and improvement in all measured growth parameters. The occurrence of both diarrheal illness and lesions typical of PPE in the challenged pigs suggest that changes in growth parameters were due to L intracellularis infection.
Nonchallenged-unmedicated pigs had significantly lower fecal scores and frequency of gross lesions of PPE compared to challenged animals. There was no histologic evidence of PPE and all ileal sections were PCR-negative for L intracellularis. This suggests that efforts to prevent cross-infection were successful.
Results of this investigation cannot necessarily be extrapolated to pigs of other age groups or pigs raised under other management of environmental conditions. A 1-week treatment period was chosen because it was considered likely to be effective under the conditions of this study, on the basis of biological plausibility. Because pigs were sacrificed on Day 17, the longer-term impacts of treatment on clinical signs, lesions, and performance cannot be determined from this investigation.
The results of this study are consistent with previously published reports of the effectiveness of tylosin in the management of PPE,4-7 and with studies of the susceptibility of L intracellularis to tylosin in vitro.13
Porcine proliferative enteropathy is recognized as a leading enteric infection of grower and finisher swine and causes significant morbidity, mortality, and economic loss to swine producers worldwide.2,14-19 Appropriate use of antimicrobials is an important component of the control of this disease.2,3,20 In this study, we used a challenge inoculum obtained from ileal mucosa of naturally infected pigs and administered by the natural route of infection. Although the study design did not permit for long-term monitoring post treatment, the results provide evidence that tylosin tartrate administered in drinking water is effective in reducing clinical signs, lesions, and suppression of growth and feed efficiency associated with PPE in swine exposed to L intracellularis.
Implication
- Under the conditions of this study, tylosin tartrate administered in drinking water to nursery pigs infected with L intracellularis is effective in reducing the clinical signs, lesions, and suppression of growth and feed efficiency associated with PPE.
References
1. Barker IK, Van Dreumel AA, Palmer N. The alimentary system. In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals. 4th ed. San Diego, California: Academic Press; 1993:1-317.
2. McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:521-534.
*3. Winkelman NL, Dee S. Ileitis: An update. Compend Contin Educ Pract Vet. 1996;18:S19-S25.
4. Veenhuizen MF, Mowrey DH, Moore GM, Watkins LE. Evaluating a natural outbreak of porcine proliferative enteropathy and treatment with tylosin in the grow-finish phase. Swine Health Prod. 1998;6:67-72.
5. McOrist S, Smith SH, Klein T. Monitored control programme for proliferative enteropathy on British pig farms. Vet Rec. 1999;144:202-204.
6. Marsteller T, Winkelman N, Gebhart C, Armbruster G, Weldon W, Muller R, Weatherford J, Symanowski J. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet Ther. 2001;2:51-60.
7. McOrist S, Morgan J, Veenhuizen MF, Lawrence K, Kroger HW. Oral administration of tylosin phosphate for treatment and prevention of proliferative enteropathy in pigs. Am J Vet Res. 1997;58:136-139.
8. Guedes RM, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:528-530.
9. Winkelman NL, Crane JP, Elfring GD, Kratzer DD, Meeuwse DM, Dame KJ, Buckham SL, Gebhart CJ. Lincomycin-medicated feed for the control of porcine proliferative enteropathy (ileitis) in swine. Swine Health Prod. 2002;10:107-111.
10. Canadian Council on Animal Care. In: Olfert ED, Cross BM, McWilliam AA, eds. Guide to the care and use of experimental animals. Vol 1. 2nd ed. Ottawa, Ontario: CCAC; 1993.
11. Steel RG, Torrie JH. Principles and procedures of statistics, a biometrical approach. New York: McGraw Hill Book Co; 1980.
12. Patience JF, Thacker PA, de Lange CFM. Swine Nutrition Guide. 2nd ed. Saskatoon, Saskatchewan: Prairie Swine Centre; 1995.
13. McOrist S, Gebhart CJ. In vitro testing of antimicrobial agents for proliferative enteropathy (ileitis). Swine Health Prod. 1995;3:146-149.
14. Wilson TM, Chang K, Gebhart CJ, Kurtz HJ, Drake TR, Lintner V. Porcine proliferative enteritis: serological, microbiological and pathological studies from three field epizootics. Can J Vet Res. 1986;50:217-220.
15. Holyoake PK, Cutler RS, Caple IW. Prevalence of proliferative enteritis on pig farms in Australia. Aust Vet J. 1994;71:418-422.
16. Christensen NH, Cullinane LC. Monitoring the health of pigs in New Zealand abattoirs. NZ Vet J. 1990;38:136-141.
17. Holyoake PK, Mullan BP, Cutler RS. Simulation of the economic impact of proliferative enteritis on pig production in Australia. Aust Vet J. 1996;73:89-92.
18. McOrist S, Smith SH, Green LE. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579-581.
19. Wilson JB, Pauling GE, McEwen BJ, Smart N, Carman PS, Dick CP. A descriptive study of the frequency and characteristics of proliferative enteropathy in swine in Ontario by analyzing routine animal health surveillance data. Can Vet J. 1999;40:713-717.
*20. McOrist S, Morgan JH, Cooper J, Carr V, Jonker L, Veenhuizen M, deRidder E. The treatment of induced porcine proliferative enteropathy (ileitis) with tylosin tartrate (Tylan soluble,, Elanco) administered via drinking water [abstract]. IPVS Cong. Birmingham, England. 1998:118. Abstract 118.
* Non-refereed references