Brief communications |
Peer reviewed |
The temporal relationship of fecal shedding of Lawsonia intracellularis and seroconversion in field cases
James Mark Hammer, DVM
Pharmacia Animal Health, Kalamazoo, Michigan; Dr J. M. Hammer, 12494 Sunset Terrace, Clive, IA 50325; E-mail: jamesmarkhammer@aol.com
Cite as: Hammer JM.. The temporal relationship of fecal shedding of Lawsonia intracellularis and seroconversion in field cases. J Swine Health Prod 2004;12(1):29-33.
Also available as a PDF.
Summary
In a cross-sectional study in nine herds, the interval between earliest detection of Lawsonia intracellularis by fecal polymerase chain reaction (at 6 to 23 weeks of age) and seroconversion (immunoperoxidase monolayer assay at 12 to 27 weeks of age) ranged from 2 to 8 weeks (mode, 2 weeks).
Keywords: swine, proliferative
enteropathy,Lawsonia intracellularis, polymerase chain reaction, immunoperoxidase
monolayer assay
Search the AASV web site
for pages with similar keywords.
Received: February
5, 2003
Accepted: August
11, 2003
Proliferative enteropathy, or ileitis, is a troubling disease for producers and veterinarians to control in the field. The causative agent is Lawsonia intracellularis (LI), an obligate intracellular bacterium.1-3 Because the bacterium cannot be cultivated using conventional methods, antemortem diagnosis and herd dynamics of LI exposure must be made on the basis of serological tests or assays for fecal shedding.
Studies using various serological techniques or polymerase chain reaction (PCR) to determine the incidence and prevalence of LI infection have been reported.4-8 When the indirect immunofluorescence antibody test (IFAT) was used, 316 to 73.3%7 of herds tested positive. Just et al9 reported seroprevalence ranging from 0 to 54% by farm site in pigs < 25 weeks of age. In this serial serological study, seroconversion occurred at 12 weeks of age, with no animals testing positive beyond 17 weeks of age.9 These studies investigated the prevalence of LI by various methodologies, but fell short of determining when LI was being shed within a herd.
The use of the immunoperoxidase monolayer assay (IPMA) has been limited to challenge model investigations10-11 and a few field studies. Guedes et al12 reported 100% specificity and 89% sensitivity at a serum dilution of 1:30 for the IPMA serological test. Guedes et al13 also determined that there was 98.6% agreement between the IFAT and the IPMA tests in experimentally infected pigs, concluding that both serological tests provide similar results .These studies show that the IPMA is a good herd level test to identify exposure to LI, but the period of fecal shedding has not been related to the time of seroconversion in field studies. To improve control strategies, practitioners must be able to use serological tests to estimate when LI is being shed in a herd, which requires an understanding of the interval between fecal shedding and seroconversion under field conditions.
Serology may be useful to swine veterinarians to elucidate the course of LI infection in a herd, but the temporal relationship of fecal shedding of LI to seroconversion needs to be understood to improve timing of control therapies, including antibiotic placement and vaccination. The objective of this study was to determine the temporal relationship of detection of fecal shedding of LI, determined by fecal polymerase chain reaction (fPCR), and IPMA seroconversion within the study herds. The impact of nursery and finishing pig flow on LI shedding and seroconversion is also examined.
Materials and Methods
Selection of study herds
Herds were enrolled on the basis of three criteria. First, each herd had a history of ileitis, diagnosed by necropsy findings with laboratory confirmation by histopathology, immunohistochemistry, or a previous positive PCR on fecal samples or tissue scrapings. Second, in each selected herd, clinical signs of ileitis had been present, including either porcine intestinal adenomatosis (PIA) or porcine hemorrhagic enteropathy (PHE). The clinical signs for PIA were defined as nonhemorrhagic diarrhea (soft or watery stools) occurring between6 and 20 weeks of age and gaunt pigs with poor performance, as indicated by dramatic weight variation between pigs in the finishing barns. The clinical signs of PHE were defined as hemorrhagic diarrhea, pale pigs with dark brown to bloody stool, and sudden death occurring between 4 to 12 months of age.14 Finally, herd managers in enrolled herds were willing to allow sample collection and share medication histories and performance records. All reported medication programs included, at some phase of production, an in-feed antimicrobial shown to have efficacy against LI. Because of the varied in-feed medication programs used in the study herds, an analysis was not possible.
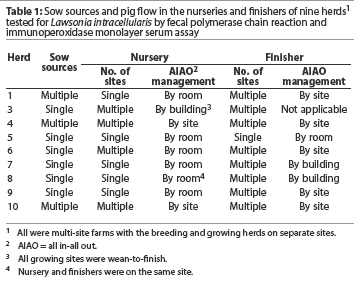
All herds were in multi-site farms with the breeding herd separated from the growing herd. Pig flow management, which varied by herd, is described in Table 1. Nurseries housed piglets from approximately 4.5 kg to approximately 25 kg (3 to 10 weeks of age). Finishers housed pigs from approximately 25 kg to market weight. Nurseries and finishers were categorized by the number of sow sources entering the nursery farm(s), all in-all out practices, and the number of sites housing pigs. Pig flow was continuous in single site facilities and noncontinuous in multiple site facilities.
Sampling and testing procedures
A cross-sectional sampling technique was used, designed to detect a 10% infection rate at a 95% confidence interval. Thirty pigs were selected for sampling from a barn or age group (cohort). The number of pens in a barn or housing a cohort was divided by 30 to determine the number of pigs sampled per pen. If more than 30 pens housed a single cohort, every other pen or every third pen was sampled as required.
Pigs were restrained for venipuncture and digital collection of feces. Pig flows were sampled at 2-week intervals from 6 weeks of age through market age. Blood and fecal samples were refrigerated and shipped to the University of Minnesota for testing as described elsewhere.15-17
To determine the youngest age of seroconversion, all sera were tested by IPMA for LI. An age group was considered to be seropositive(ie, seroconverted) if any serum sample in the cohort was positive by the IPMA at the standard 1:30 serum dilution.
Fecal samples from all cohorts in a pig flow were not tested by fPCR. When the IPMA was positive in a cohort of pigs, all fecal samples from this cohort and the two younger cohorts were tested. A cohort was determined to be shedding LI if one sample was fPCR-positive. Fecal PCR testing continued in younger cohorts until no samples in a cohort were fPCR-positive. The youngest fPCR-positive cohort (ie, age at initial detection of fecal shedding of LI) was defined as the first fPCR-positive cohort in a pig flow preceded by an fPCR-negative cohort.
Herd comparison procedures
The herds were divided into two categories based on the rate of IPMA seroconversion. Seroconversion rate for a herd was classified as high if > 20% of the pigs in one or more cohorts were seropositive; seroconversion rate was classified as low to moderate if <= 20% of the pigs in all seropositive cohorts were seropositive. Herds with high and low-to-moderate seroconversion rates were compared on the basis of veterinary classification and pig flow management from the sow farm through the nursery and finishing barns. The veterinary classification of the herd was derived from the herd veterinarian’s clinical diagnosis, which was based on the cited definitions of clinical ileitis, and conversations with the herd veterinarian.
Results
Nine herds were included in the study. Age of the youngest cohort at seroconversion, age of the youngest fPCR-positive cohort, age at earliest fecal shedding of LI detected, and interval between earliest fPCR detection and seroconversion are summarized for each herd in Table 2. First evidence of seroconversion varied among herds. In herds that seroconverted to LI, age at seroconversion ranged from 12 weeks to 27 weeks. In five of the eight herds in which seroconversion was detected, one serum sample was positive in the group designated as youngest cohort at seroconversion. In one herd, seroconversion was not detected.
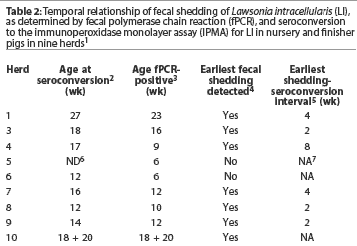
Fecal shedding of LI was detected by fPCR in all herds. The age of the youngest fPCR-positive cohort ranged from 6 weeks to 23 weeks. In the herd in which seroconversion was not detected, fPCR testing was performed on the basis of the herd veterinarian’s clinical observation and the fact that pooled fecal samples had been previously positive by fPCR in the early nursery. In this herd, fecal shedding occurred in cohorts 6 and 8 weeks of age. In two herds, age at earliest detection of fecal shedding was not identified because the youngest pigs sampled (6 weeks of age) were fPCR-positive. Excluding these two herds, the initial detection of positive fPCRs occurred in cohorts ranging in age from 9 to 23 weeks.
The interval between earliest detection of positive fPCRs and IPMA seroconversion in each herd was the difference between the ages of the youngest cohort at seroconversion and the youngest cohort that became fPCR-positive. In one herd, this interval could not be determined because pigs from different weeks of production were housed in the same barn and sampled as one cohort. The sampling of two cohorts as one in this herd confounds the determination of the interval between earliest detection of positive fPCRs and seroconversion, because the youngest cohort at seroconversion and the youngest fPCR-positive cohort were both identified in the mixed group. Excluding this herd and the herd in which seroconversion was not detected, the interval between earliest detection of positive fPCRs and seroconversion ranged from 2 to 8 weeks, with a mode of 2 weeks.
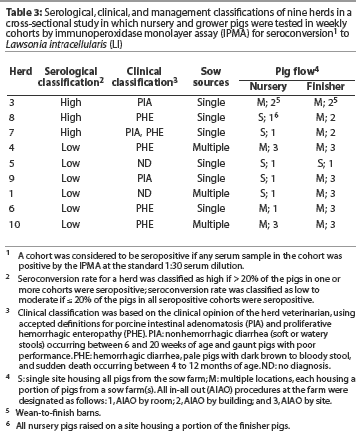
Table 3 presents the serological categories, the herd veterinarian’s classification of disease caused by LI (ie, PIA, PHE, or both), and pig flow in the nursery and finisher for each herd. Three herds (Herds 3, 7, and 8) were included in the high seroconversion category. The remaining six herds were in the low-to-moderate seroconversion category. The serological response tended to be low when pigs were segregated by site in the nursery or finisher. Herds with more age groups on a site tended to have a high serological response, even though the pigs were segregated by building or room.
Discussion
These results must be interpreted carefully because of the cross-sectional collection of the samples. Even though the same animals were not collected at different ages, the characteristics of fecal shedding and seroconversion may help practitioners understand the temporal relationship between fecal shedding of LI and seroconversion. Just et al9 theorized that a cross-sectional serological study would be the best method for determining exposure to LI in a production system. The results of the study in these nine herds may be used to draw some conclusions about the usefulness of IPMA serological testing and fPCR in determining the herd dynamics of LI infection.
The youngest cohort at seroconversion varied among herds from 12 weeks to 27 weeks of age, suggesting that exposure occurs in the late nursery period through finishing and that exposure builds until the dose of LI becomes high enough to induce seroconversion. This conclusion is supported by the fPCR results, ie, cohorts in various herds were fPCR-positive between 6 and 23 weeks of age. It has been reported that fPCR detects 103 or more LI organisms per gram of feces;15,18 therefore, not all infected pigs shed enough LI organisms in their feces to be detectable by fPCR.19
Confounding the serological results and the fPCR results are the in-feed antimicrobial programs. Use of in-feed antimicrobials may have affected the duration of the fecal shedding of LI and the age of seroconversion. In-feed antimicrobials may prevent or delay infection with LI,20 potentially delaying seroconversion. Other reports have speculated that fecal shedding, as determined by fPCR, is reduced with antimicrobial treatment.5,8 In this study, in-feed antimicrobial programs might have reduced the degree and the extent of LI shedding, potentially delaying seroconversion.
In herds where earliest shedding of LI was detected, the interval between earliest detection of positive fPCRs and IPMA seroconversion in each herd ranged from 2 to 8 weeks, with a mode of 2 weeks. On the basis of the results from the small sample of herds in this study, variation in the interval between earliest detection of shedding and seroconversion may make it difficult, using serology alone, to predict the age at which fecal shedding occurs in a herd.
The low seroconversion rate detected in some herds in this study also limits the use of serology alone in predicting the age at which fecal shedding of LI is likely to occur. Sampling intensity and the characteristics of the IPMA may contribute to the low seroconversion rate detected. The sampling intensity used to detect earliest age at seroconversion was designed to detect a 10% infection rate at a 95% confidence interval.21 In 62.5% of herds in which seroconversion was detected, one serum sample was positive in the group designated as youngest cohort at seroconversion. The sensitivity of the IPMA (89%)13 might be a factor in the low seroconversion rate in this study because of the possibility of false negatives. Nonetheless, when the first pigs in a herd seroconvert, it is likely, on the basis of these results, that fecal shedding of LI began 2 to 8 weeks previously, and timing of antibiotic treatment or vaccination for LI in the herd should be based on this estimate.
Knittel et al,22 using a pure culture challenge model and the IFAT, reported seroconversion at 14 days post challenge and PCR-positive fecal samples at 7 days post challenge. In this cross-sectional study, there was a longer shedding-to-seroconversion interval that varied from herd to herd. This variability may be related to pathogen load, antimicrobials used, testing accuracy, pig flow, or other risk factors.
In herds housing multiple cohorts of pigs, the shedding-to-seroconversion interval was less variable and the seroconversion rate was higher than in herds housing fewer cohorts of pigs. This suggests a longer but less intense exposure to LI at a younger age in herds with low-to-moderate seroconversion rates compared to herds categorized as having a high seroconversion rate.
If the temporal relationship of fecal shedding (detected by PCR) and seroconversion (detected using the IPMA) is known, practitioners can better understand and control LI transmission within a farm. With some limitations, serological testing provides an estimate of the age when LI is being shed and causing widespread exposure in a herd. Use of this knowledge to more accurately schedule antimicrobial placement and vaccination in a herd will improve control of LI.
Implications
- Under field conditions, serological testing for LI on a large scale is necessary to detect seroconversion in the youngest cohort of growing pigs.
- Results of serological testing may be used to estimate the age at which fecal shedding of LI occurs in growing pigs, but the estimate must be interpreted cautiously.
- In this cross-sectional study in nine herds, the interval between the youngest age at detection of fecal shedding of LI by PCR and seroconversion (IPMA) ranged from 2 to 8 weeks.
- Although fPCR provides a better estimate of the age at which LI is being shed in a herd, compared to serological testing, concurrent use of the IPMA and fPCR provides the best evidence of fecal shedding and exposure to LI within a herd.
Acknowledgements
I wish to thank the Pharmacia Animal Health (PAH) swine business unit management team for funding the project. Special thanks to Drs Connie Gebhart and Roberto Guedes for protocol development, laboratory services, and general encouragement during the project. A final thank you to the PAH swine technical service team of Drs James Bradford, Roberta Alvarez, Michael Senn, and Shelley Stanford for assistance throughout the project.
References
1. Jones GF, Ward GE, Murtaugh MP, Rose R, Gebhart CJ. Relationship between Ileal symbiont intracellularis and porcine proliferative enteritis. Infect Immun. 1993;61:5237-5244.
2. McOrist S, Gebhart C, Boid R, Barns S. Characteristics of Lawsonia intracellularis gen. Nov., sp. Nov., the obligate intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820-825.
3. McOrist S, Jasni S, Mackie RA, MacIntyre N, Neef N, Lawson GH. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun. 1993;61:4286-4292.
4. Just SD, Thoen CO, Thompson JU, Thacker BJ, Marstellar TA. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commercial swine production system. J Swine Health Prod. 2001;9:57-61.
*5. Pozo J, Collins AM, Rubio P, Love RJ. Maternal immunity in Lawsonia infection. Proc Int Pig Vet Soc. Melbourne, Australia. 2000;108.
*6. Hurtado ME, Maestro R, Rolo M, Palencia L, Sandoval J. Seroepidemiological evaluation of Lawsonia in swine farms in Venezuela. Proc Int Pig Vet Soc. Melbourne, Australia. 2000;28.
*7. Wendt M, Bontiz A, McOrist S. Prevalence of Lawsonia infection in German breeding herds. Proc Int Pig Vet Soc. Melbourne, Australia. 2000;27.
*8. Ohlinger VF, Pesch S, Knittel, J. Prevalence of Lawsonia intracellularis in diagnostic samples from Germany, the Netherlands and Belgium. Proc Int Pig Vet Soc. Melbourne, Australia. 2000;70.
*9. Just SD, Thoen CO, Thompson JU, Thacker BJ, Marstellar TA. Monitoring of Lawsonia on a commercial swine farm. Proc AASP. Des Moines, Iowa. 1999;27.
*10. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA, Marsteller TA. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Proc Allen D. Leman Swine Conf. St Paul, Minnesota. 2000;70-72.
*11. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA. Comparative study of an indirect immunofluorescent test and the immunoperoxidase monolayer assay for diagnosing porcine proliferative enteropathy. Proc Int Pig Vet Soc. Melbourne, Australia. 2000; 61.
12. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA. Comparative study of an indirect immunofluorescent test and the immunoperoxidase monolayer assay for diagnosing porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:420-423.
13. Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serological test for porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:528-530.
14. McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. 1999;524-525.
15. Jones GF, Ward GE, Gebhart CJ, Murtaugh MP, Collins JE. Use of a DNA probe to detect the intracellular organism of proliferative enteritis in swine feces. Am J Vet Res. 1993;54:1585-1590.
16. McOrist S, Gebhart CJ, Lawson GH. Polymerase chain reaction for diagnosis of porcine proliferative enteropathy. Vet Microbiol. 1994;41:205-212.
17. Guedes RM, Gebhart CJ, Winkelman NL, Mackie-Nuss RA, Marsteller TA, Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can J Vet Res. 2002;66:99-107.
18. Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993;31:2611-2615.
19. McOrist S, Boid R, Lawson GHK, McConnell I. Monoclonal antibodies to intracellular Campylobacter-like organisms of the porcine proliferative enteropathies. Vet Rec. 1987;121:421-422.
*20. Collins AM, Van Dijk M, McOrist S, Love RJ. Strategic medication and development of immunity to Lawsonia. Proc Int Pig Vet Soc. Melbourne, Australia. 2000;30.
21. Cannon RM, Roe RT. Livestock Disease Surveys: A Field Manual for Veterinarians. Canberra, Australia: Australian Government Publishing Service. 1982;16.
22. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am J Vet Res. 1998;59:722-726.
* Non-refereed references.