Case report |
Peer reviewed |
Methods of control and elimination of porcine reproductive and respiratory syndrome virus using modified live vaccine in a two-site production system
Thomas G. Gillespie, DVM, Diplomate ABVP; Amy L. Carroll
Cite as: Gillespie TG, Carroll AL. Methods of control and elimination of porcine reproductive and respiratory syndrome virus using modified live vaccine in a two-site production system. J Swine Health Prod. 2003;11(6):291-295.
Also available as a PDF.
TGG: Rensselaer Swine Services, Rensselaer, Indiana; ALC: Purdue University, West Lafayette, Indiana; Corresponding author: Thomas Gillespie, 210 East Wood Rd, Rensselaer, IN 47978; Tel: 219- 866-6465; Fax: 219-866-3431; E-mail: tom.gillespie@rssvet.com.
Summary
A control and elimination program for porcine reproductive and respiratory syndrome virus (PRRSV) was initiated in a two-site, 1250-female herd with a primary business plan of supplying replacement females to commercial herds. Modified live virus (MLV) PRRS vaccine was used to control field PRRSV, which then allowed total virus elimination from all populations. First, the adult sow population was mass vaccinated, and the sow herd was closed for less than 3 months. When PRRSV-negative offspring were being produced,the nursery was depopulated and pigs at the nursery-finisher site were vaccinated with MLV vaccine. After the herd was "stabilized" (ie, exhibited no horizontal or vertical PRRSV transmission), PRRS-positive and previously exposed animals were gradually removed, and a naive herd was established. Polymerase chain reaction and ELISA serological testing for PRRSV were performed on a sample of the population statistically selected to detect 5% prevalence at a 95% confidence level. However, the elimination program deviated from the original plan by allowing use of field-infected finisher animals as replacement gilts. The group of infected gilts used was monitored closely and replaced with naive replacement gilts when profitable market prices returned. Statistical process control techniques were utilized in the serologic monitoring program.
Keywords: swine, porcine reproductive
and respiratory syndrome virus, elimination, modified live vaccine
Search the AASV web site for pages with similar keywords.
Received: September 27, 2002
Accepted: May 7, 2003
Porcine reproductive and respiratory syndrome (PRRS) has played a large role in the United States swine industry over the past several years. It has been ranked by producers as the second most commonly encountered disease problem in the breeding herd1 and costs the global swine industry great financial losses annually.
Due to the financially devastating effects incurred due to production losses associated with PRRS in both the reproductive and growth phases, there has been an increasing demand by commercial producers to purchase replacement gilts naive for PRRS virus (PRRSV), ie, gilts from herds in which PRRSV has not been identified or isolated, and in which animals have repeatedly tested PRRS-seronegative. This has required the implementation of PRRSV-elimination programs in previously PRRSV-positive replacement gilt genetic multiplier units (ie, operations with a primary business plan of supplying replacement females to commercial herds).2,3 Published elimination programs have used methods that include herd closure,2,3 mass vaccination with modified live virus (MLV) vaccine,4,5 temporary off-site weaning,5 partial depopulation,6 test-and-remove programs,7 and deliberate exposure to field virus2 to control PRRSV activity within a described population of animals, with the goal of ultimately eliminating PRRSV in that population. The use of MLV vaccines in addition to other supporting management techniques may improve the success rate of control and elimination programs, as well as minimizing disruption in production.
There is a tremendous financial advantage for a swine production operation, especially a genetic multiplier, to maintain a PRRSV-naive swine population. Probably the most important economic advantages of eliminating PRRSV are the increased productivity and efficiency that can be attained when this virus is not present. In addition to the enhanced reproductive performance of the sow herd and growth of the finisher pigs, more aggressive piglet fostering techniques may be implemented in the farrowing rooms as a result of lower disease pressures, so that more and better pigs are weaned. For multiplier herds, successful elimination of PRRSV allows a much greater opportunity for replacement gilt sales.
When discussing a PRRSV-elimination project, several terms are used that may be interpreted ambiguously. For the purposes of this report, the following definitions will be used. "Naive" refers to an animal or population of animals that has neither been exposed to PRRSV nor possessed PRRSV antibody due to passive immunity. A "negative animal" is one that has been previously exposed to PRRSV, but has experienced antibody decay such that serological ELISA testing (IDEXX HerdChek; Idexx Laboratories, Westbrook, Maine) yields a sample:positive (S:P) ratio <0.4. An animal may also be described as negative if it has received maternal antibody to PRRSV, but this antibody level has subsequently decayed until the ELISA S:P ratio is <0.4. A "negative population" of animals is defined by testing a subsample of the population statistically selected to detect a 10% seroprevalence at a 95% confidence level. All tested animals must have an ELISA S:P ratio <0.4 for the population to be considered negative. A" stable herd" is defined as one in which clinical signs of PRRS have not been observed and horizontal or vertical shedding of PRRSV has not been detected by serological ELISA or polymerase chain reaction (PCR) testing of sentinel animals. A "sentinel animal" is an animal that is naive to PRRSV and is introduced into a stable population as a sensitive indicator of horizontal virus spread. The sentinel animal is known to have no PRRSV antibody prior to introduction into the herd; therefore, seroconversion in this animal indicates exposure to PRRSV in the population being tested.
Case description
A control-and-elimination program was undertaken at a two-site replacement gilt multiplier unit that had experienced an outbreak of clinical PRRS. The program implemented on this farm was based on the usage of MLV PRRSV vaccine, unidirectional animal flow, strict biosecurity practices, and strategic PCR testing and ELISA serological testing (HerdChek PRRS ELISA). The PCR test was used for early detection of virus activity.
This herd consisted of 1250 sows located at the sow site, which housed all sows and unweaned pigs. All females were bred by artificial insemination using semen from a PRRSV-naive boar stud. Sows were housed in crates during breeding and gestation.
Pigs were weaned at an average of 18 to 20 days of age and were moved to the nursery-finisher site a few miles away. This site consisted of one building with eight nursery rooms, each housing 500 pigs, and eight finisher rooms, each housing 1000 pigs. Pigs from two nursery rooms moved together into a single finisher room. Pigs were housed in the nursery for approximately 50 days and in the finisher room for 100 to 112 days. All rooms were managed all in-all out. Females in the finisher stage of production that met specific criteria were sold as replacement gilts to commercial farms. The remaining gilts and barrows were sold to market.
The objectives of this project were two-fold. The initial goal was to control field PRRSV within the herd and stabilize the sow herd by using MLV vaccine. After stabilization of the herd, the second step was to eliminate the virus and discontinue vaccination in all stages of production. The ultimate goal resulting from these two objectives was to have only PRRSV-naive animals in the herd.
This herd experienced a clinical PRRS outbreak in November 1998, characterized by late term abortions, weak pigs at birth, and high preweaning mortality. Virus isolation and ELISA tests confirmed PRRSV activity associated with the clinical signs. Forty sows tested by ELISA were seropositive, with S:P ratios ranging from 0.5 to 3.7. The PRRSV was isolated from piglets exhibiting clinical signs and had a predicted restriction fragment length polymorphism pattern of 1-1-1.
The entire sow herd, including all adult animals in all stages of production (approximately 1250 head), was simultaneously vaccinated with an MLV PRRSV vaccine (Ingelvac PRRS MLV; Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri)immediately after the initial diagnostic tests confirmed PRRSV activity, and again 30 days later, to stimulate a homologous immune response (Table 1). No clinical signs were present 30 days after the initial vaccination. Herd S:P ratios were monitored monthly by ELISA testing of 30 selected adult females of different ages, with the first test 60 days after the second vaccination. Herd S:P ratios had remained stable or declined after several monthly samples, indicating no additional exposure to PRRSV. Initially, all efforts were designed to control virus activity in the sows (ie, to stop abortions and improve piglet health at birth by using MLV vaccine), thereby initiating stabilization of the adult population and terminating occurrence of clinical signs. Approximately 90 days after the last observation of clinical signs, offspring produced from the sow herd were PRRSV-negative, and a flow of negative pigs into the nursery-finisher site was established. Each month, 30 pigs were randomly selected from different nursery rooms for PCR testing, and samples were pooled (three samples per pool) for testing. All results were negative.
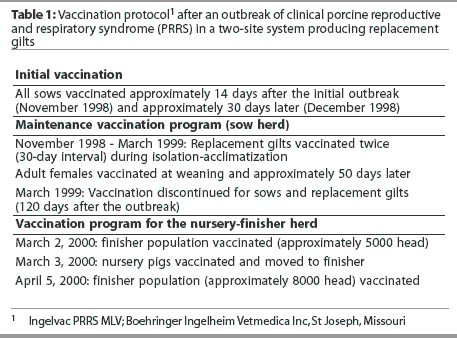
As a maintenance program, between December 1998 and March 1999, all replacement animals were isolated in an on-site separate building prior to entry into the breeding herd and were vaccinated twice during the isolation and acclimatization period. Females in the breeding herd continued to be vaccinated at weaning and at midgestation. By mid-March, vaccination was discontinued for replacement animals and adult females already in the breeding herd. The herd was closed, with no new gilt introductions between May and July 1999. This was primarily the result of market conditions and financial constraints, but it provided adequate time for the vaccinated animals to develop immunity and decreased the risk of PRRSV shedding after vaccination was discontinued.
Due to cost restraints and a change in the source of replacement animals, desired naive replacement gilts were unavailable for purchase. It was decided to use internally raised gilts from the herd’s PRRSV-exposed finisher unit as replacement animals. Studies had demonstrated that PRRS-positive, infected, unvaccinated gilts may be successfully incorporated into the breeding herd without compromising herd PRRSV stability, provided that exposure to PRRSV occurs when the gilts are young (Scott Dee, oral communication, June 1999). This approach was adopted in the case herd by introducing gilts from the finisher site into the sow herd. Monthly ELISA testing of 10 pigs per finisher room, beginning in May 1999, confirmed that seroconversion to field PRRSV occurred in finisher pigs at 80 to 100 days of age, which was less than a month after placement in the finisher room. After this early exposure, 180 gilts in the finisher were tested at approximately 145 days of age, then 120 of these gilts were selected for proper conformation and were retested twice during the isolation-acclimatization period. When the same animal was tested three times, the ELISA S:P ratio declined with time. Sentinel animals placed in the isolation building with the group of 120 infected, unvaccinated gilts did not seroconvert (Table 2). It has been documented that 4-month-old animals infected with PRRSV do not shed virus to age-matched sentinels 90 days post infection.8 In this case, selected replacement gilts remained in the finisher barn until 180 days of age, and were then housed in the isolation-acclimatization facilities for a minimum of 60 days. Therefore, these animals were not introduced into the breeding herd for at least 160 days after initial PRRSV exposure. Although these animals had been previously infected with PRRS field virus, they were considered stable prior to introduction into the breeding herd. Seronegative offspring (the youngest pigs in the nursery) were used as sentinels for these gilts during the isolation-acclimatization period to detect shedding of PRRSV.
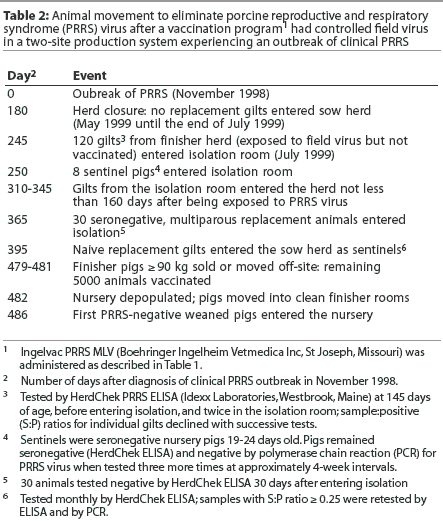
Due to the lack of availability of naive replacement gilts, a few naive weaned sows were introduced into the herd from a new source farm in November 1999. Naive replacement gilts approximately 180 days of age were introduced immediately after these few naive weaned sows. In all groups of 120 naive replacement animals, 30 were tested by ELISA approximately 30 days after they entered the isolation room. Once these animals entered the sow herd, they became sentinels to detect horizontal PRRSV transmission. All available sentinel animals in the herd were tested by ELISA monthly until October 2000, when there were enough sentinels in the herd to test 40 new sentinels per month. This testing protocol detects 10% prevalence with a 95% confidence level. No seroconversion of sentinel animals has been detected for more than 3 years after replacement gilts were first introduced.
In February 2000, 30 suckling piglets (one piglet from each of 30 litters) were tested for PRRSV by PCR. In addition, 30 nursery pigs were tested by ELISA. All test results were negative, indicating that there had been no vertical virus transmission of PRRSV from the sows. However, seroconversion was still occurring in the finisher. After it was determined that the sow herd had been stabilized and offspring remained negative until they were placed in the finisher, the focus of the program turned toward elimination of PRRSV in the nursery-finisher site. All finishing pigs weighing 90 kg or more were either sold or moved off-site, emptying three of the eight finisher rooms. The remaining finisher pigs (approximately 5000 animals) were mass vaccinated with Ingelvac PRRS MLV. Thirty days later, with approximately 8000 head in the finishers, a second dose of Ingelvac PRRS MLV was administered. All nursery rooms were depopulated, and the pigs were moved to the recently emptied finishing spaces. This created a break in pig flow prior to introduction of PRRSV-negative pigs into the nurseries. The nurseries were thoroughly cleaned and disinfected, and the first PRRSV-negative pigs entered the nursery complex within 4 days after it had been depopulated. Rooms that had been empty for 2 weeks or longer were rewashed and disinfected just prior to filling in order to provide a clean environment.
By July 2000, all exposed, PRRS-positive animals had been removed from the finisher through normal marketing, leaving only seronegative animals at the nursery-finisher site. Monthly ELISA testing continued, including 40 sows and 50 finishers (10 animals from each of five different finisher rooms). Samples with ELISA S:P ratios >=0.25 were retested both by ELISA and PCR, in order to rule out false-positive results and to detect an early infection.
Naive replacement animals continued to be introduced into the sow herd, while animals that had been previously infected, vaccinated, or both during the original outbreak of field virus were gradually marketed. In 1999, females that had been infected, vaccinated,or both could not be marketed quickly. Contributing to this were poor market prices and low profitability, lack of a source of naive replacement gilts, and isolation facilities limited to housing <=120 animals. All exposed adult animals had been eliminated from the herd 3 years after the initiation of the elimination program. A summary of the vaccination protocol and animal movement after the initial PRRSV outbreak can be found in Tables 1 and 2.
Discussion
Statistical process control (SPC) charting was utilized to monitor PRRSV ELISA S:P ratios as well as herd production parameters. This served as a production tool for monitoring and moving forward with continual improvement. Specifically, SPC charting analyzes variation in data to determine if significant signals are produced by the production and serological data, or if variation is due to routine deviation from the mean. The upper and lower critical limits, defined as standard deviations from the mean, were recalculated when a production change was started. The first recalculation occurred on the first occasion when only naive replacement animals were ELISA tested. The second recalculation occurred when all females that were infected, vaccinated, or both were removed through marketing. Although no significant signals were detected when SPC charting was applied to the serological results, production parameters did exhibit signals a few times. Signals from production issues are caused by numerous influences, primarily season and employee changes. Signals concerning production issues were never a result of PRRSV activity. Over time, the use of SPC charting added confidence that the elimination program was progressing successfully. As there continues to be no detection of PRRSV in any production phase of this herd, the elimination program was deemed successful.
Elimination programs for PRRSV must be structured to the capabilities of the farm personnel, facility design, strain virulence, and the farm’s goal. This elimination project demonstrated that an MLV PRRSV vaccine combined with management techniques controlled one field strain of PRRSV from circulating in a large two-site swine operation. Management techniques included mass vaccination of populations of animals, pig flow changes, and an intense serological monitoring program. Although PRRS field virus activity may subside without use of an MLV vaccine, vaccine was used in this case to support the management techniques, to assure complete success in as short a time period as possible. Sound management practices and strict biosecurity protocols were utilized to prevent movement of PRRSV from positive to negative populations during the control and elimination program.2-4,6,7 Extensive herd monitoring for PRRSV activity by both PCR and ELISA testing was used throughout the elimination program. Testing by PCR is useful to determine exposure of nursing piglets to PRRSV shedding by the sow. Pigs become viremic within 24 hours post inoculation9 and therefore are PCR-positive much earlier than they become seropositive. A positive ELISA titer cannot be detected until at least 10 days post inoculation.10 Various production stages were monitored monthly during the elimination program in order to ensure initial control and eventual elimination of PRRSV. Statistical process control charting of both production and serologic results, in addition to monitoring for clinical signs, were in place throughout the entire program. Ultimately, monthly monitoring using ELISA and PCR testing and marketing of all animals that have been infected, vaccinated,or both, determines when the elimination program has been successful.
On this farm, some unique situations and challenges affected the progression of the elimination program. There was a need to select replacement gilts from infected, unvaccinated gilts in the finisher when a source of naive gilts was not available and the market price was extremely low. The isolation-acclimatization room proved to be especially valuable in holding the infected,home-raised gilts longer than the normal 60 days, to allow for sufficient immunity to develop.8 This temporary change in the program proved to be a solution for the economic situation faced at the time, while maintaining the herd’s status to PRRSV. The naive replacement animals served an important role as sentinels in the monitoring program. With the ever increasing demand for PRRSV-negative animals,veterinarians must become familiar with elimination programs that result in production of PRRS-naive pigs that may be used as replacement animals.
Implications
- Use of MLV PRRSV vaccine supported management techniques in controlling field PRRSV activity in populations of females and finisher pigs on different sites in a two-site system, thereby producing seronegative offspring.
- If PRRSV-elimination programs are to be successful, they must be designed specifically and flexibly for each facility and production unit, and all parts of the program must be established before any part is initiated.
- Prolonged removal of adult females exposed to PRRSV, vaccinated for PRRS, or both, need not compromise a PRRSV-elimination program.
- Options for PRRS testing for proper diagnosis and routine monitoring, such as PCR and serological ELISA, must be utilized strategically and with samples selected to provide statistically significant results.
- Sound management practices and biosecurity protocols contribute to the success of a PRRSV-elimination program.
References – refereed
1. United States Department of Agriculture. Part II: Reference of Swine Health and Health Management in the United States: 2000. Fort Collins, Colorado: United State Department of Agriculture: Animal and Plant Health Inspection Service: Veterinary Services: Centers for Epidemiology and Animal Health, National Animal Health Monitoring System; 2002. Publication N355.0202.
2. Desrosiers R, Boutin M. An attempt to eradicate porcine reproductive and respiratory syndrome virus (PRRSV) after an outbreak in a breeding herd: eradication strategy and persistence of antibody titers in sows. J Swine Health Prod. 2000;10:23-25.
3. Torremorell M, Moore C, Christianson W. Establishment of a herd negative for porcine reproductive and respiratory syndrome virus (PRRSV) from PRRSV-positive sources. J Swine Health Prod. 2002;10:153-160.
4. Dee SA, Joo HS, Park BK, Molitor TW, Bruna G. Attempted elimination of porcine reproductive and respiratory syndrome virus from a seedstock farm by vaccination of the breeding herd and nursery depopulation. Vet Rec. 1998;142:569-572.
7. Dee SA, Bierk MD, Deen J, Molitor TW. An evaluation of test and removal for the elimination of porcine reproductive and respiratory syndrome virus from 5 swine farms. Can J Vet Med. 2001;65:22-27.
8. Batista L, Dee SA, Rossow KD, Deen J, Pijoan C. Assessing the duration of persistence and shedding of porcine reproductive and respiratory syndrome virus in a large population of breeding age gilts. Can J Vet Res. 2002;66:196-200.
9. Spagnuolo-Weaver M, Walker IW, McNeilly F, Calvert V, Graham D, Burns K, Adair BM, Allan GM. The reverse transcriptase polymerase chain reaction for the diagnosis of porcine reproductive and respiratory syndrome: comparison with virus isolation and serology. Vet Microbiol. 1998;62:207-215.
10. Lamontagne L, Page C, Larochelle R, Longin D, Magar R. Polyclonal activation of B cells occurs in lymphoid organs from porcine reproductive and respiratory syndrome virus (PRRSV)-infected pigs. Vet Immunol Immunopathol. 2001;82:165-181.
References – non refereed
5. Dee SA, Philips RC. Using vaccination and unidirectional pig flow to control PRRSV transmission. Swine Health Prod. 1998;6:21-25.
6. Philips RC, Dee SA, Karhoff K. Elimination of the PRRS virus from a farrow to finish single site farm using mass vaccination and unidirectional pig flow strategy. Proc AASP. St Louis, Missouri. 1999:113-1157.