Original research |
Peer reviewed |
Pilot studies to evaluate the efficacy of a truck-mounted tire sanitizer system
Sandra F. Amass, DVM, PhD, Diplomate ABVP; Jessica L. Schneider, RVT; Darryl Ragland, DVM, PhD; Michael A. Hill, PhD, MRCVS
National Biosecurity Resource Center, Purdue University School of Veterinary Medicine, West Lafayette, Indian. Corresponding author: Dr Sandra F. Amass, National Biosecurity Resource Center, Purdue University School of Veterinary Medicine, VCS/Lynn, 625 Harrison Street, West Lafayette, IN 47907-2026; Tel: 765-494-8052; Fax: 765-496-2608; E-mail: amasss@purdue.edu.
Cite as: Amass SF, Schneider JL, Ragland D, et al. Pilot studies to evaluate the efficacy of a truck-mounted tire sanitizer system. J Swine Health Prod. 2003;11(6):277-283.
Also available as a PDF.
Summary
Objective: To determine whether using a tire sanitizer system to apply a disinfectant or water would reduce or eliminate bacteria on the tires of a truck after it was driven through a swine farm.
Materials and methods: After truck tires were contaminated by driving on a farm road in the spring and fall, disinfectant or water was sprayed on the left tires using a tire sanitizer system. The right tires were untreated controls. Bacterial counts were compared from swabs collected from standard areas of the tire after contamination and after treatment.
Results: Use of the tire sanitizer system with disinfectant was effective in removing bacteria from the tire footprint and, in most cases, the tread grooves of tires in fall and spring seasons. Use of the tire sanitizer system to spray water was not effective in reducing bacterial counts. Driving along an asphalt road, without use of disinfectant, removed bacteria from tires in some cases. In cool, muddy conditions, bacterial counts on the tires were lower when the tire sanitizer system was used than when the system was not used, but counts were not consistently lower in warm, dry conditions.
Implications: Bacterial numbers on tires varied with temperature and moisture conditions. Use of the tire sanitizer system with disinfectant was effective in removing bacteria from the footprints and, in most cases, from the tread grooves. Use of tire sanitizers with disinfectant may be an adjunct tool in protocols for vehicle decontamination.
Keywords: swine, tire, decontamination, vehicle, biosecurity
Search the AASV web site for pages with similar keywords.
Received: January 6, 2003
Accepted: August 11, 2003
Most biosecurity policies address the issue that vehicles driven within and among pork production units can mechanically spread swine pathogens. Vehicle contamination has been implicated as a source of Actinobacillus pleuropneumoniae1 and suggested as a source of Salmonella serovars2,3 and Escherichia coli3 under natural conditions (ie, the vehicles were not intentionally inoculated with an organism and then sampled). Cleaning and disinfection of vehicles reduces levels of both Salmonella organisms and Escherichia coli on the vehicles.3Many farms require that vehicles be cleaned and disinfected before entering the premises. Peer-reviewed procedures for vehicle cleaning and disinfection are detailed and time-consuming.4-6 Consequently, in many cases, either vehicle cleaning and disinfection measures are not used, or quick protocols are substituted (eg, for veterinary trucks, feed trucks, and sales representative cars) due to cost, time constraints, and feasibility of daily, multiple vehicle washes.
Recently, tire sanitizers were introduced to the pork, poultry, and dairy industries in the United States and the United Kingdom. Tire sanitizer systems are sprayers mounted in the wheel wells of vehicles. Prior to farm entry or after farm exit, the driver can automatically spray disinfectant on the tires for 15 seconds to 1 minute. Of course, the rest of the vehicle remains contaminated, but the tires, which have ground contact, supposedly are sanitized. Although tire sanitizers are not marketed as cleaners and are intended to supplement whole-vehicle cleaning, they are often used on uncleaned tires.
There are no peer-reviewed published reports on the effect of tire sanitizer use. The objective of this study was to determine whether using a tire sanitizer to apply either a peroxygen compound or water would reduce or eliminate aerobic bacteria from the tires of a veterinary truck after it was driven on roads of a pork production unit.
Material and methods
Experiment One: Tire sanitizer used with disinfectant (fall)
Environment. Sampling for this experiment was performed during a 3-hour period on the premises of the Purdue Animal Sciences Research and Education Center Swine Unit in Montmorenci, Indiana,on November 4, 2002. Temperature and relative humidity were 4.7°C and 73.7%, respectively, at the beginning of the sampling period, and 8.2 degrees C and 42.5%, respectively, at the end of the sampling period(VelociCalc Plus 8360 digital temp/RH/velocity meter; TSI Incorporated, St Paul, Minnesota). Rain had fallen the previous day and evening, but did not fall during the sample collection period. Farm roads were a mixture of gravel, grass, soil, and mud. Main roads were asphalt.
Equipment. A 1994 half-ton pickup truck with a short bed (Ford F150; Ford Motor Company, Detroit, Michigan) was equipped with a mobile unit (Crown Jewel; Porta-Vet, Hudson, Iowa ) and a tire sanitizer system (On-Board Tire Sanitizer; Monroe Snow and Ice Control, Monroe, Wisconsin). Front tires were identical tubeless radial tires (Firestone Supreme P235/75R15/M+S; Bridgestone/Firestone Americas Holding Inc, Nashville, Tennessee) with four-ply treads (two polyester and two steel) and two-ply polyester sidewalls. The water tank of the mobile unit was modified so that liquid could be supplied to either the mobile unit or the tire sanitizer system. The tank was filled with a peroxygen compound (Virkon S; Farnam Livestock Products,Phoenix, Arizona). Two spray nozzles were mounted on each wheel well. When the sanitizer system was activated, disinfectant was sprayed at 45 psi onto the tread grooves, footprint (part of the tread that contacts the road), and sidewalls of the left front tire (Treatment). A cut-off valve prevented disinfectant from being sprayed onto the right front tire (Negative Control). Rear tires were equipped in the same fashion; however, rear tires were not sampled during Experiment One. When activated via an in-cab start button, the tire sanitizer system dispensed 0.47 L of disinfectant per nozzle for 15 seconds.
Experimental design. Immediately prior to the study, the truck was cleaned in a commercial drive-through car wash and was then driven to the parking lot of the swine farm. At the farm, disinfectant was dispensed to the right front tire for a total of 30 seconds to clear dispenser lines. Ten replicates of the following protocol were then performed. Front tires were hosed with farm water for 3.5 to 4 minutes to remove all visible disinfectant residue, organic material, and gravel. Tires were contaminated by driving the truck on farm roads at speeds of up to 44 km per hour in a figure-eight pattern to ensure contact of both front tires with similar road substrate. Briefly, the truck was driven for 0.64 km clockwise, then 0.64 km counterclockwise, and finally, 0.48 km clockwise to the junction of the farm road and the main road. Contaminated tires were sampled as described below. Next, the truck was driven off farm premises directly onto an asphalt road. Disinfectant dispensing began when all four tires contacted the asphalt road, and continued for two consecutive 15-secondintervals. The truck was driven on the asphalt road at approximately 32 km per hour for the first 15-second interval. Braking occurred during the last 15-second interval, and the truck was stopped approximately5 seconds before disinfectant dispensing ended. Post-treatment samples were collected immediately; thus, in the period after disinfectant dispensing ceased and before sampling, tires collected no additional organic material from the road.
Sampling procedure. After contamination and after treatment, a standardized area of the footprint of each front tire (approximately 4.06 cm2), was sampled using a sterile polyester-tipped applicator swab. Additionally, a 0.855 cm3 volume of the tread groove and its contents were sampled after contamination and after treatment using a sterile polyester-tipped applicator swab. The sampling areas on each tire were identified in each replicate to avoid resampling the same area. This prevented initial swab samples from removing bacteria that should have remained on the tire for subsequent swab samples.
Experiment Two: Tire sanitizer used with water (spring)
Environment. Sampling for this experiment was performed during a 3.5-hour period on the premises of the Purdue Animal Sciences Research and Education Center Swine Unit on May 8, 2003. Temperature and relative humidity were 11.9°C and 80.8%, respectively, at the beginning of the sampling period, and 15°C and 72.5%, respectively, at the end of the sampling period (VelociCalc Plus 8360 digital temp/RH/velocity meter). Weather conditions were cloudy and dry. Farm roads were a mixture of gravel, grass, and soil. Main roads were asphalt.
Equipment. The same truck, equipped with the same mobile unit and sanitizer system, was used as in Experiment One. However, rear tires were sampled instead of front tires, because the original front tires on the truck had been replaced with a different brand and tread pattern. Two spray nozzles were mounted on each rear wheel well. When the sanitizer system was activated, water was sprayed at 45 psi onto the tread grooves, footprint, and sidewalls of the left rear tire (Treatment). The right rear tire was equipped identically; however, a cut-off valve prevented water from being sprayed onto this tire (Negative Control). Front tires were equipped in same fashion; however, front tires were not sampled during Experiment Two.
The water tank of the mobile unit was initially filled with hose water from the Purdue University School of Veterinary Medicine. When a sample of water from the tire sprayer nozzle was cultured aerobically, there was no growth (0 colony-forming units [cfu] per mL). During replicate 7, water from the swine unit was added after the sprayer became obstructed with debris and had to be flushed. When a sample of this water from the tire sprayer nozzle was cultured, the aerobic bacterial count was 20 cfu per mL. The sprayer was repositioned using duct tape after the filters and spray nozzles had been disconnected for flushing.
Experimental design. The truck was driven to the parking lot of the swine farm. Ten replicates of the following protocol were then performed. Rear tires were hosed for 1 minute each with water from the swine farm (bacterial count, 200 cfu per mL when a water sample from the hose nozzle was cultured aerobically) to remove all visible organic material and gravel. Tires were contaminated by driving the truck on farm roads at speeds of up to 37 km per hour in a figure-eight pattern to ensure contact of both rear tires with similar road substrate. Briefly, the truck was driven for 0.64 km clockwise, then 0.64 km counterclockwise, and finally, 0.16 km clockwise to the junction of the farm road and the main road. Contaminated tires were sampled as described. Next, the truck was driven off farm premises directly onto an asphalt road. Water dispensing began when all four tires contacted the asphalt road, and continued for two consecutive 15-secondintervals. The truck was driven on the asphalt road at approximately 32 km per hour for the first 15-second interval. Braking occurred during the last 15-second interval, and the truck was stopped approximately 5 seconds before water dispensing ended. Post-treatment samples were collected immediately; thus, in the period after water dispensing ceased and before sampling, tires collected no additional organic material from the road.
Sampling procedure. A standardized area of the footprint of each rear tire (approximately 6.05 cm2) was sampled after contamination and after treatment using a sterile polyester-tipped applicator swab. Additionally, a 0.875-cm3 volume of the tread groove and its contents were sampled after contamination and after treatment using a sterile polyester-tipped applicator swab.
Experiment Three: Tire sanitizer used with disinfectant (spring)
Environment. Sampling for this experiment was performed during a 2.5-hour period on the premises of the Purdue Animal Sciences Research and Education Center Swine Unit on May 14, 2003. Temperature and relative humidity were 16.1°C and 63%, respectively, at the beginning of the sampling period, and 18°C and 57.9%, respectively, at the end of the sampling period (VelociCalc Plus 8360 digital temp/RH/velocity meter). Weather conditions were cloudy. Extremely light rain had fallen just prior to the study, but this had ceased before the study began. Farm roads were a mixture of gravel, grass, and soil. Main roads were asphalt.
Equipment. The same truck, equipped with the same mobile unit and sanitizer system, was used as in the previous experiments, and rear tires were sampled as in Experiment Two. When the sanitizer system was activated, disinfectant was sprayed at 45 psi onto the tread grooves, footprint, and sidewalls of the left rear tire (Treatment). A cut-off valve prevented disinfectant from being sprayed onto the right rear tire (Negative Control). Front tires were equipped in the same fashion; however, front tires were not sampled during Experiment Three.
Experimental design. The truck was washed in a commercial truck wash and driven to the parking lot of the swine farm. Ten replicates of the following protocol were then performed. Rear tires were hosed for 1 minute each with water from the swine farm (bacterial count, 28,000 cfu per mL when a water sample collected from the hose nozzle was cultured aerobically) to remove all visible organic material and gravel. No aerobic bacteria (0 cfu per mL) were cultured from a sample of disinfectant from the tire sprayer. Tires were contaminated by driving the truck on farm roads at speeds of up to 35.2 km per hour in a figure-eight pattern to ensure contact of both rear tires with similar road substrate. Briefly, the truck was driven for 0.64 km clockwise, then 0.64 km counterclockwise, and finally, 0.16 km clockwise to the junction of the farm road and the main road. Contaminated tires were sampled as described. Next, the truck was driven off farm premises directly onto an asphalt road. Disinfectant dispensing began when all four tires contacted the asphalt road, and continued for two consecutive 15-secondintervals. The truck was driven on the asphalt road at approximately 32 km per hour for the first 15-second interval. Braking occurred during the last 15-second interval, and the truck was stopped approximately 5 seconds before disinfectant dispensing ended. Post-treatment samples were collected immediately; thus, in the period after disinfectant dispensing ceased and before sampling, tires collected no additional organic material from the road.
Sampling procedure. A standardized area of the footprint of each rear tire (approximately 6.05 cm2) was sampled after contamination and after treatment using a sterile polyester-tipped applicator swab. Additionally, a 0.875-cm3 volume of the tread groove and its contents were sampled after contamination and after treatment using a sterile polyester-tipped applicator swab.
Preparation of disinfectant for Experiments One and Three
Twelve gallons (45.6 L) of a 2% solution of Virkon S (Farnam Livestock Products, Phoenix, Arizona) were prepared according to label directions. Briefly, 2.6 ounces (72.8 g) of Virkon S powder was added to each gallon (3.8 L) of water.
Culture methods for Experiments One, Two, and Three
Swab samples were placed in individual sterile tubes containing 2 mL of sterile chemical broth to inactivate residual disinfectant (D/E Neutralizing Broth; Becton-Dickenson, Franklin Lakes, New Jersey). The swab portion was broken off into the tube of broth and the contents were mixed by hand agitation. Samples were placed on cold packs in a cooler on the farm and during transport, then refrigerated until processing. Prior to dilution and culture, all samples were vortexed briefly (Experiment One) or mixed by hand agitation (Experiments Two and Three). A 100-mL aliquot of each original sample was plated directly onto 5% sheep blood agar. Additionally, serial tenfold dilutions were made using sterile D/E Neutralizing Broth, and a 100-mL aliquot of each dilution was plated directly onto 5% sheep blood agar. Samples were incubated at 37°C for 24 to 26 hours in Experiment One, and for 24 hours for Experiments Two and Three. Colonies of aerobic bacteria were counted and total aerobic bacteria counts were calculated. Attempts were not made to identify specific pathogens.
Data analysis for Experiments One, Two, and Three
Mean and median bacterial counts before and after treatment were compared. Graph Pad InStat version 3.00 for Windows 95 (GraphPad Software, San Diego, California) was used for statistical calculations. The software package used in this study tested the data sets in this experiment for normality using the method of Kolmogorov and Smirnov to calculate the KS statistic (GraphPad Software). The P value for the normality test was computed by the software using the Dallal and Wilkinson approximation to Lilliefors’ method (GraphPad Software). The interpretation of a large P value is that the data is consistent with a Gaussian population. All data sets in Experiments One, Two, and Three passed the normality test at P >.10. However, the test for normality is not always accurate for sample sizes less than 12. Thus, the data sets were analyzed using tests that assumed nonparametric data. A Mann-Whitney test was used to compare median bacterial counts between tires before and after treatment. A Wilcoxon matched-pairs signed-ranks test was used to determine whether the median of the differences between bacterial counts on the same tire before and after treatment differed significantly from zero. For all tests, P <.05 was considered statistically significant.
Data analysis assuming normality was also performed, but results have not been reported due to space limitations. These results did not change the overall conclusions and are available from the corresponding author upon request.
Results
Experiment One: Tire sanitizer used with disinfectant (fall)
A mixture of gravel, soil, and mud was visible on the tires after the truck had been driven on farm roads.
Footprint. Median bacterial counts are presented in Table 1. The median number of bacteria cultured per 4.06-cm2 area of the footprint after tires were contaminated did not differ between tires. Spraying the tire for 30 seconds with disinfectant reduced the median number of bacteria cultured. The median number of bacteria cultured was also reduced on untreated tires. The median number of bacteria cultured from the footprint was lower for tires sprayed with disinfectant than for untreated tires.
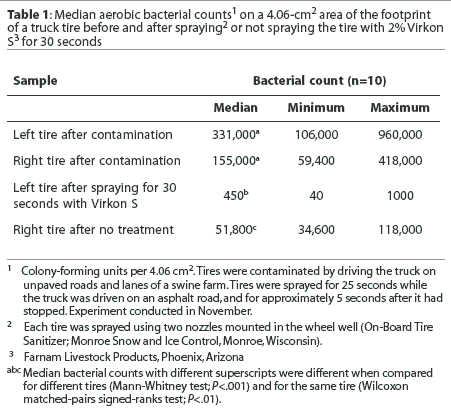
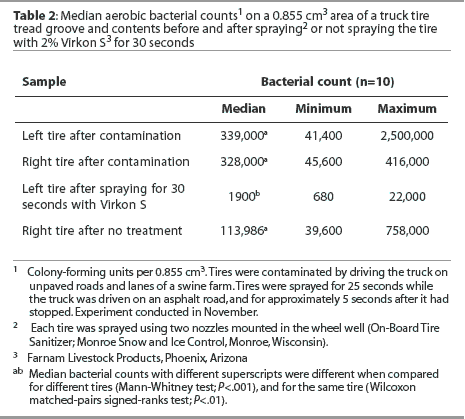
Tread groove. Median bacterial counts are presented in Table 2. The median numbers of bacteria cultured per 0.855 cm3 volume of the tread groove after tires were contaminated did not differ between tires. The median number of bacteria cultured was reduced for tires sprayed with disinfectant for 30 seconds, but not for untreated tires. Fewer bacteria were cultured from the tread groove of tires sprayed with disinfectant compared to untreated tires.
Experiment Two: Tire sanitizer used with water (spring)
A mixture of gravel, soil, and dirt was visible on the tires after the truck had been driven on farm roads.
Footprint. Median bacterial counts are presented in Table 3. The median number of bacteria cultured per 6.05-cm2 area of the tire footprint was less for the right tire than the left after tires were contaminated. The median number of bacteria cultured was not reduced either in tires sprayed with water for 30 seconds or in untreated tires. However, the median number of bacteria cultured from the footprint was less for untreated tires compared to tires sprayed with water.
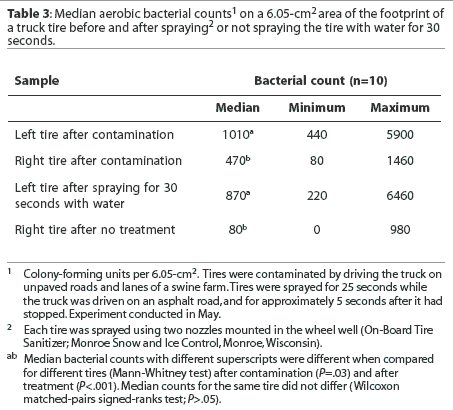
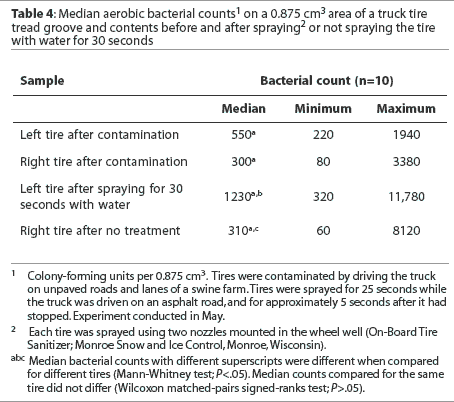
Tread groove. Median bacterial counts are presented in Table 4. The median numbers of bacteria cultured per 0.875 cm3 volume of the tread groove after tires were contaminated did not differ between tires. The median number of bacteria cultured was not reduced either in tires sprayed with water for 30 seconds or in untreated tires. Fewer bacteria were cultured from the tread groove when tires were untreated than when tires were sprayed with water.
Experiment Three: Sanitizer used with disinfectant (spring)
A mixture of gravel, soil, and dirt was visible on the tires after the truck had been driven on farm roads.
Footprint. Median bacterial counts are presented in Table 5. The median numbers of bacteria cultured per 6.05-cm2 area of the footprint after tires were contaminated did not differ. The median number of bacteria cultured was reduced both when the tire was sprayed with disinfectant for 30 seconds and when the tire was untreated. However, the median number of bacteria cultured from the footprint was less for untreated tires than for tires sprayed with disinfectant.
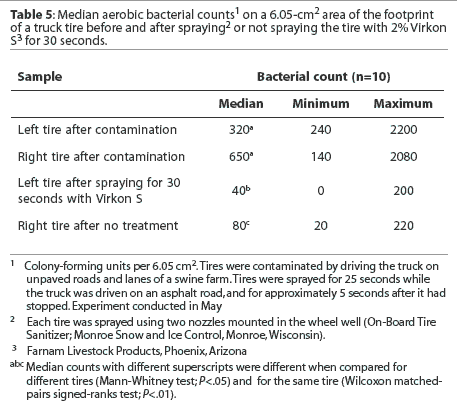
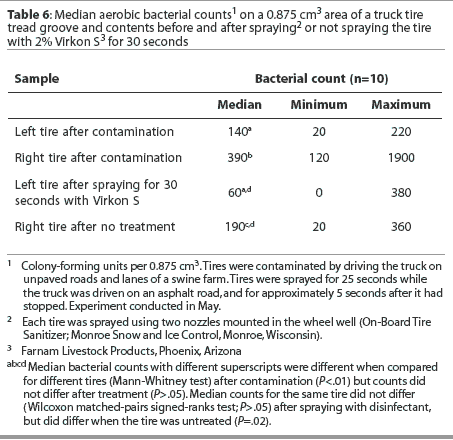
Tread groove. Median bacterial counts are presented in Table 6. Fewer bacteria were cultured per 0.875-cm3 volume of the tread groove of the left tire compared to the right tire at the start of the experiment. The median number of bacteria cultured was not reduced after tires were sprayed with disinfectant for 30 seconds, but was reduced when tires were untreated. The median number of bacteria cultured from the tread groove did not differ between treated and untreated tires.
Discussion
Contaminated vehicles are potential vectors for mechanical transmission of swine pathogens; however, time constraints and feasibility often result in uncleaned vehicles moving among farms. The tires in this study were contaminated under natural field conditions on a typical Midwestern farm. Experiments were performed during fall and spring days under conditions that should reflect actual field conditions during a farm visit. The three independent studies cannot be compared due to differences in timing. However, a seasonal effect on the number of bacteria cultured from tires at the start of the study was noted, with bacterial counts being approximately 100-fold higher during cool, muddy fall conditions compared to warm, dry spring conditions. Differences in the initial bacterial counts might have affected the study outcomes.
The results of Experiment One demonstrated that using a tire sanitizer system with a peroxygen disinfectant may reduce the number of aerobic bacteria on the footprint and tread grooves of truck tires under cool, muddy fall conditions. When the tire sanitizer was not used, the number of aerobic bacteria was reduced on the footprint but not in the tread groove of the tires after the truck was driven a short distance along an asphalt road. Reduction of bacterial counts on the untreated footprint was presumably due to friction and heat generated at the tire-road interface. Fewer aerobic bacteria were cultured from both footprints and tread grooves of tires treated using the tire sanitizer system, compared to untreated tires. Presumably, the spray action of the tire sanitizer system facilitated removal of organic material, and subsequently bacteria, from both the footprint and tread grooves. Therefore, the low pressure spray (45 psi) had some merit, although the recommended pressure for vehicle decontamination is 700 psi.6
The results of Experiments Two and Three were different from those of Experiment One, presumably due to the warm, dry spring conditions. The results of Experiment Three were similar to those of Experiment One in that there was a reduction in the number of bacteria present on the tire footprint regardless of whether or not the tire was sprayed with disinfectant. Additionally, the number of bacteria on the tire footprint was significantly lower after the tire was sprayed with disinfectant. In Experiment Three, the number of bacteria in the tread groove was significantly different between left and right tires at the beginning of the experiment, and adequate comparisons could not be made. The authors cannot explain these differences, as due care was taken during all experiments to expose the tires to the same substrate.
In Experiment Two, the tire sanitizer sprayed water on the tires instead of disinfectant, under warm, dry spring conditions. There was no reduction in the number of bacteria on the tire footprint whether or not water was sprayed on the tires. Moreover, fewer bacteria were cultured from the tire footprint of untreated tires. Presumably, the moisture enhanced contamination of the footprint with organic material, as in Experiment One, when muddy conditions were associated with high bacterial counts. Similarly, there was no reduction of bacterial counts in the tread groove whether or not water was sprayed on the tires. Fewer bacteria were cultured from the tread groove of untreated tires compared to tires sprayed with water. The results of Experiments Two and Three offer limited data to demonstrate that disinfectant is needed to reduce the bacterial counts on the footprint of the tire, and that the spray action of the tire sanitizer is not sufficient to physically remove bacteria.
Böhm4 reported that 103 cfu per cm2 is a general target for the number of aerobic bacteria to be cultured from a surface after cleaning and prior to disinfection. In Experiment One, the tire sanitizer "cleansed" the footprint of the tire in all 20 replicates using this criterion. In Experiment Two, the bacterial counts immediately after contamination would have been classified as "clean" in 50% and 70% of the left and right tire footprint samples, respectively, and in 70% and 60% of the left and right tread groove samples, respectively. The percentage of footprint bacterial counts in the "clean" category remained at 50% after the tire was sprayed with water, and increased to 100% when the tire was untreated. Similarly, the percentage of tread groove samples in the "clean" category was reduced to 40% after spraying with water and increased to 70% in untreated tires. In Experiment Three, the bacterial counts immediately after contamination would have been classified as "clean" in 90% and 60% of the left and right tire footprint samples, respectively, and in 100% and 90% of the left and right tread groove samples, respectively. All footprint and tread groove samples were classified as "clean" both after spraying with disinfectant and after no treatment.
Tamási7 defined successful disinfection as the culture of <=1 viable aerobic bacterium per cm2 of the treated surface. In Experiment One, disinfection of the footprint or tread groove of tires was not accomplished after spraying the tire with disinfectant. In Experiment Two, the only sample classified as "disinfected" was a single footprint sample from an untreated tire. In Experiment Three, 20% of footprint samples and 10% of tread groove samples were classified as "disinfected" after the tire was sprayed with disinfectant. No footprint or tread groove samples from untreated tires were classified as "disinfected." The criteria for cleaning and disinfection could not be used to evaluate tread grooves, because there is no direct relationship between volume (cm3) and area (cm2).
As tire sanitizer systems were being used with disinfectant in the field, Experiment One was designed to determine the benefits of a tire sanitizer system used with disinfectant, and to compare the culture results for untreated tires and tires treated with disinfectant delivered by a tire sanitizer system. Thus, our negative control, to represent trucks not equipped with the tire sanitizer, was no treatment, ie, no liquid was sprayed on the tire. The authors did not attempt to determine whether the treatment effect was due to the use of disinfectant or the spray action of the tire sanitizer during cool, muddy fall conditions. Experiments Two and Three, respectively, tested spraying of water or disinfectant by the sanitizer in warm, dry spring conditions. Spraying the tire with water appeared to increase bacterial counts compared to no treatment. Presumably, the moisture enhanced bacterial contamination of the tire. For unknown reasons, sprayers and filters repeatedly became obstructed when water alone was used in the tire sanitizer system. As a result, it was necessary to remove obstructions repeatedly between replicates to get 10 replicates in which the tire sanitizer functioned correctly. Obstruction of sprayers and filters did not occur when Virkon S was used in the system. The authors do not recommend using water alone in tire sanitizer systems at this time; however, future research will test whether or not spraying tires with water is as effective in reducing bacterial counts as spraying with disinfectant under cold or muddy conditions or both. The valve diaphragms in the tire sanitizer system malfunctioned after Experiment One, presumably due to contact with the disinfectant. These diaphragms were replaced with materials resistant to disinfectant and no further problems were noted in Experiment Three.
Use of the tire sanitizer has limitations. First, the remainder of the vehicle remains contaminated even though bacterial numbers may be reduced on the tires. Thus, contaminated organic material may still fall from other parts of the vehicle (eg, the interior,bed, body, wheel wells, or undercarriage) and potentially transmit infectious agents. The tire sanitizer does not replace whole vehicle decontamination. Second, the list price for the on-board sanitizer tested in this study was US$2238, which might be cost prohibitive for some indications. However, the benefit of enhanced public perception after viewing tire sanitizers on veterinary trucks or other farm vehicles might prove advantageous and outweigh the financial impact of purchase and installation. Third, temperature and weather conditions appear to affect the need for a tire sanitizer system. Warm, dry conditions may negate the need for a tire sanitizer system. Finally, the soil type on the farm used to test the tire sanitizer in this study was silt loam and silt clay loam. Soil composition might affect the distribution of bacteria within the soil.8 The soil itself might also act as a barrier to prevent disinfectant from reaching the bacteria. Thus, the tire sanitizer might have varying efficacy depending on soil type.
Research is in progress to further test the tire sanitizer. Future research will address the efficacy of different disinfectants under varying environmental and seasonal conditions. Further examination of modifications to the mobile unit and tire sanitizer, combining features of both systems to facilitate use, is ongoing.
Implications
- Use of the tire sanitizer system to spray 2% Virkon S for 30 seconds was effective in reducing the number of bacteria from the footprint, and in all but one case, the tread grooves of truck tires contaminated by contact with farm roads.
- Use of the tire sanitizer system to spray water for 30 seconds did not reduce the number of bacteria from the tires.
- In most cases, driving < 0.16 km along an asphalt road without use of disinfectant reduced the number of bacteria on the footprint of the tire, the tread groove of the tire, or both.
- The tire sanitizer may not be needed in warm, dry conditions.
- The tire sanitizer may be utilized as one of the many tools needed to achieve full vehicle biosecurity.
- Use of the tire sanitizer alone is not sufficient to decontaminate other areas of the vehicle that can serve as sources of infectious organic material and therefore is not a substitute for whole vehicle decontamination.
Acknowledgments
The investigators thank the American Association of Swine Veterinarians Foundation for funding this project. The investigators also thank Monroe Liquid Systems, Leann McGowan, Tom Schartner, and Allen Peterson for donating and installing the tire sanitizer system and for their technical assistance during the project. The investigators thank Gordon Moser of PortaVet for use of the mobile unit, which was modified to enable sharing of the water tank between the PortaVet unit and the tire sanitizer. Finally, the investigators thank Josh Morgan for his assistance during the project.
References – refereed
1. Fussing V, Barfod K, Nielsen R, Møller K, Nielsen JP, Wegener HC, Bisgaard M. Evaluation and application of ribotyping for epidemiological studies of Actinobacillus pleuropneumoniae in Denmark. Vet Microbiol. 1998;62:145-162.
2. Fedorka-Cray PJ, Hogg A, Gray JT, Lorenzen K, Velasquez J, Von Behren P. Feed and feed trucks as sources of Salmonella contamination in swine. Swine Health Prod. 1997;5:189-193.
3. Rajkowski KT, Eblen S, Laubauch C. Efficacy of washing and sanitizing trailers used for swine transport in reduction of Salmonella and Escherichia coli. J Food Prot. 1998;61:31-35.
4. Böhm R. Disinfection and hygiene in the veterinary field and disinfection of animal houses and transport vehicles. Int Biodeterioration Biodegradation. 1998;41:217-224.
5. Poumian AM. Disinfection of trucks and trailers. Rev sci tech Off Int Epiz. 1995;14:171-176.
6. Ford WB. Disinfection procedures for personnel and vehicles entering and leaving contaminated premises. Rev sci tech Off Int Epiz. 1995;14:393-401.
7. Tamási G. Testing disinfectants for efficacy. Rev sci tech Off Int Epiz. 1995;14:75-79.
8. Alexander M. Biodegradation and Bioremediationnd ed. San Diego, California: Academic Press; 1999.