Original research |
Peer reviewed |
Efficacy of prophylactic tilmicosin in the control of experimentally induced Haemophilus parasuis infection in pigs
Janet I. MacInnes, BSc, PhD; Marie-Anne Paradis, DVM; Gordon H. Vessie, Diplomate APM; Ljubomir Slavic, DVM; Sheila Watson, BSc; Jeffrey B. Wilson, DVM, DVSc, PhD; Jeffery J. Aramini, DVM, MSc, PhD; C. Paul Dick, DVM, MSc
JIM, LS, SW: Department of Pathobiology, University of Guelph, Guelph, Ontario. MAP, GHV, CPD: Elanco, Division of Eli Lilly Canada, Guelph, Ontario. JBW, JJA: Department of Population Medicine, University of Guelph, Guelph, Ontario. Corresponding author: Dr Marie-Anne Paradis, Elanco, Division of Eli Lilly Canada, 1050 du Seminaire Blvd N, Suite 200, St-Jean-sur-Richelieu, Quebec J3A 1S7; Fax: 450-349-9541; E-mail: paradis_marie_anne@lilly.com.
MacInnes JI, Paradis MA, Vessie GH, et al. Efficacy of prophylactic tilmicosin in the control of experimentally induced Haemophilus parasuis infection in pigs. J Swine Health Prod. 2003;11(4):174-180. Also available as a PDF
Summary
Objective: To evaluate the effect of in-feed tilmicosin on the morbidity, mortality, and production parameters of pigs experimentally challenged with a virulent field strain of Haemophilus parasuis.
Methods: The study consisted of three replicates of a trial in which 19- to 22-day-old pigs were randomly assigned to one of four treatment groups (nine pigs per group) on Day -4: unchallenged-unmedicated, challenged-unmedicated, challenged-tilmicosin 200 mg per kg, and challenged-tilmicosin 400 mg per kg. Challenged groups were randomly assigned to one of three rooms. The unchallenged group was assigned to the same room for the three replicates to minimize the potential for H parasuis cross-contamination. Medicated feed was provided ad libitum from Day 0 to trial termination (Day 21). Aerosol challenge occurred on Day 7 with a virulent H parasuis serotype 5 isolate. Outcomes assessed included mortality, gross lesions and culture of H parasuis at necropsy, clinical scores, and growth parameters.
Results: Among challenged pigs, compared to unmedicated pigs, treatment with tilmicosin at 200 mg per kg of feed reduced the frequency of gross lesions consistent with H parasuis infection, culture of H parasuis, and H parasuis-specific mortality (P<.05), and improved clinical scores and growth parameters (P<.05), except for feed:gain. In addition, a significant trend (P<.05) towards further improvements in all parameters with increasing tilmicosin dose (400 mg per kg) was demonstrated by linear regression.
Implications: Under the conditions of this study, tilmicosin was effective in controlling H parasuis infection in weaned pigs challenged by aerosol inoculation with a virulent field strain.
Keywords: swine, Haemophilus
parasuis, Glasser’s disease, tilmicosin, antibiotic
Search for similar articles
Received: June 7, 2002
Accepted: November 21, 2002
Once associated with a sporadic disease in young pigs character-ized by polyserositis and arthritis (Glasser’s disease), Haemophilus parasuis has emerged as a major swine pathogen. In addition to polyserositis, H parasuis is increasingly associated with respiratory disease and septicemia.1-5 The introduction of H parasuis, particularly into high-health-status herds, may result in systemic disease of high morbidity and mortality affecting swine at any stage of production.3,6 Disease induced by H parasuis has been associated with the mixing of swine from different herds and with the introduction of new breeding stock.3,6 The increasing importance of H parasuis has been attributed, in part, to the growing frequency of high-health-status herds.1-3,6
Appropriate use of antimicrobials is considered to be an important component of the management of H parasuis infection. Because H parasuis may kill pigs very rapidly, prompt treatment is recommended.1-4 Oral antibiotics have also been recommended for herds in which H parasuis is a problem, when pigs have to be handled or mixed or when naive replacement gilts or boars are introduced.2 The absence of a clinically relevant and reproducible experimental model for H parasuis infection has hampered the identification of clinically effective antimicrobial agents.
Tilmicosin, a chemically modified macrolide with demonstrated efficacy in the in vitro inhibition of field strains of H parasuis,7 has proven efficacy in reducing the severity of porcine respiratory disease associated with Actinobacillus pleuropneumoniae and Pasteurella multocida.8-10 The objective of this study was to evaluate the efficacy of tilmicosin in the control of H parasuis infection in weaned pigs challenged by aerosol inoculation with a virulent field strain.
Materials and methods
Study animals
A total of 108 high-health-status pigs were used. Pigs were between 23 and 26 days of age on Day 0 of the study. On the basis of historical health records, the source herd (the Arkell herd at the University of Guelph) was stable for porcine reproductive and respiratory (PRRS) virus and negative for A pleuropneumoniae, Brachyspira hyodysenteriae, Salmonella serovars, transmissiblegastroenteritis virus, Mycoplasma hyopneumoniae, mange, lice, and H parasuis. In order to confirm that the herd was negative for H parasuis, prior to trial commencement, nasal and tonsillar swabs collected from six pigs on three different occasions were plated on a selective medium containing pleuropneumoniae-like organism (PPLO) agar without crystal violet (Difco, Sparks, Maryland), with bacitracin (5 µg per mL), lincomycin (1.5 µg per mL), crystal violet (0.1 µg per mL), and 0.02% nicotinamide adenine dinucleotide (NAD). Plates were incubated for 48 hours at 37°C in an atmosphere of 5% CO2.
Housing and feeding
Throughout the study, pigs were housed in four rooms (nine pigs per room). Each room measured 5 x 3 m and had solid concrete floors. The lighting program, heating, ventilation, and other management procedures were typical of modern swine farms in Ontario. Prior to the trial, pigs received unmedicated creep feed. Between Day -4 and Day 0, an unmedicated starter feed was introduced. Medicated starter feed was provided as described, beginning on Day 0. Feed was provided ad libitum in one trough-type feeder (100 cm of feeding space) per room. Water was provided ad libitum by one nipple drinker per room.
Treatment groups
The study consisted of three replicates, with 36 pigs in each replicate randomly assigned to four groups: challenged-unmedicated (n=9); challenged-tilmicosin 200 mg per kg (n=9); challenged-tilmicosin 400 mg per kg (n=9); and unchallenged-unmedicated controls (n=9). Challenged treatment groups were randomly assigned to three adjacent rooms. The unchallenged-unmedicated control group was assigned to the same room (separated from the other three rooms by one empty room) for all three replicates to minimize the potential for cross-contamination with H parasuis. Tilmicosin (Pulmotil; Elanco Animal Health, Eli Lilly and Co, Indianapolis, Indiana) was commercially mixed with feed to the specified concentrations (200 and 400 mg per kg of feed) prior to each replicate. Feed was provided ad libitum. Pigs that were injured or developed clinical illness between Days -4 and Day 0 were removed from the trial.
Study design
A randomized complete block design was used with replicates (n=3) corresponding to blocks. All analyses were conducted at the room level. Treatment (medicated feed) was provided from Day 0 to the end of the trial (Day 21); challenge with H parasuis occurred on Day 7.
Challenge organism
A virulent H parasuis serotype 5 isolate, HP1185, from a pig with Glasser’s disease, was used as the challenge organism. Strain HP1185 was cultured on PPLO agar without crystal violet (Difco) with 0.01% NAD at 37°C in an atmosphere of 5% CO2. For each challenge, one aliquot of glycerol seed stock was used to inoculate one plate of PPLO-NAD. After 24 hours of growth, six colonies were selected and used to heavily streak two PPLO-NAD plates. At 18 hours, cells were harvested from the surface of these plates in 5 mL of phosphate buffered saline (PBS), then diluted in PBS to a final concentration of approximately 1 x 105 colony forming units (CFU) per mL.
Challenge with H parasuis
On Day 7, pigs were challenged in a closed metal chamber equipped with a nebulizer (DeVilbis, Model 65, Summerset, Pennsylvania). The chamber consisted of a five-sided metal box (120 x 120 x 75 cm) with a Plexiglas lid. Pigs were placed in the chamber nine at a time (three pigs from each challenged group). A 50-mL suspension of H parasuis HP1185 (approximately 1 x 105 CFU per mL) was aerosolised for 10 minutes at the highest flow setting in the chamber. As the chamber volume was relatively large, air additional to that emitted by the nebulizer was not provided. Pigs remained in the chamber for an additional 10 minutes following nebulization. Unchallenged control pigs received a placebo treatment of PBS. In order to minimise the possibility of contamination, the placebo treatment was undertaken first.
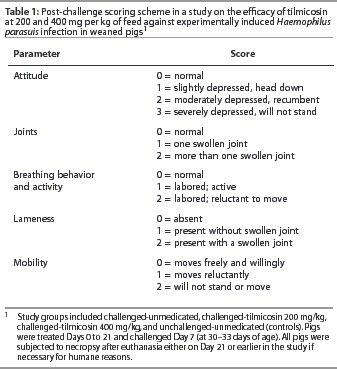
Observations
Individual pigs were observed daily. After challenge (Day 7), on Days 7 through 21, a daily clinical score was recorded for each pig for the parameters listed in Table 1, with a total possible daily score of 11 for each pig. For humane reasons, pigs with a daily clinical score of 7 or higher were humanely euthanised. All pigs were individually weighed on Days -4, 0, 7, and 21. Feed weights for individual rooms were recorded, and uneaten feed was weighed and recorded as needed during the trial.
Necropsy
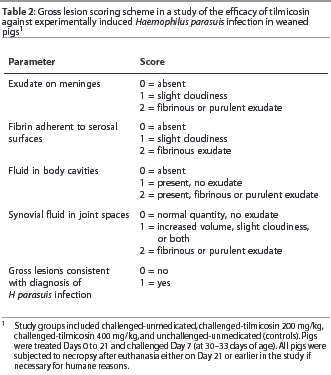
Pigs euthanised for humane reasons prior to Day 21, and all remaining pigs that were euthanised at trial termination (Day 21), were subjected to routine post mortem examination. The pleural, pericardial, and peritoneal cavities, meninges, and all shoulder, elbow, carpal, hip, stifle, hock, and atlanto-occipital joints in each pig were examined and scored as summarized in Table 2. The pleural, pericardial, and peritoneal surfaces, the basal meninges, trachea, and both hocks, stifles, carpal joints, and elbows were swabbed and cultured on PPLO-NAD. A maximum of two additional joints (ie, in addition to those swabbed above) that were opened and contained fibrinous or purulent exudate were also swabbed.
Criteria for identification of H parasuis
The following criteria were used to identify H parasuis recovered at post-mortem: small gram-negative rod, often in chains, forms non-pigmented to slightly yellow colonies approximately 2 mm in diameter on PPLO-NAD plates; grows on blood agar containing NAD but does not grow without NAD; nonhaemolytic, CAMP negative, urease-negative, mannitol-negative, and glucose-positive. Cultures were classified as either positive or no growth. Selected isolates were also serotyped (Gallant Custom Laboratories, Guelph, Ontario).
Calculations
Pig-days were calculated for each pig and consisted of the number of days each pig contributed to the study (from Day 0 to euthanasia). Daily gain per pig was defined as body weight gain (from Day 0 to euthanasia) divided by animal-days. Daily feed intake per pig was calculated as the total weight of feed consumed divided by the sum of pig-days for individual animals for each room. To analyze clinical scores, a composite clinical score variable was created that consisted of the sum of the individual daily clinical scores (attitude, joints, breathing behaviour, lameness, and mobility) for each pig, divided by the number of pig-days. Mean individual pig scores were then averaged for each room.
For analysis of gross lesion data, each pig was categorized as having gross lesions consistent with H parasuis infection or not. In order to be categorized as having gross lesions consistent with H parasuis infection, pigs had to have a score of 2 for at least one of the four locations indicated in Table 2 (ie, meninges, serosal surfaces, body cavities, joints).
Prior to analysis, parameters measured as a proportion (p = x/y) at the room level (ie, mortality, lesions, and post-mortem isolation of H parasuis) were subjected to an empirical logit transformation of the form logit(x) = log [(x + 0.1) / (y – x) + 0.1].
Statistical analysis
All analyses were conducted at the room level. Using analysis of variance (ANOVA), the following outcomes were used to test the effect of treatment: mean clinical score; mean final body weight; average daily gain; mean feed:gain; mean total feed intake; and the proportions of pigs (transformed as previously described) with gross lesions consistent with H parasuis infection, post-mortem isolation of H parasuis, spontaneous mortality or humane euthanasia due to all causes (excluding euthanasia at trial termination), and spontaneous mortality or humane euthanasia with lesions and culture consistent with H parasuis (excluding euthanasia at trial termination). For all outcomes examined, comparisons were made, using ANOVA, between unchallenged-unmedicated and challenged-unmedicated pigs, and among the dose levels of tilmicosin (unmedicated, 200 and 400 mg per kg) for challenged pigs. For the latter, pair-wise t-tests among treatment means were undertaken provided that the overall F test was significant (P <=.05). The effect of tilmicosin dose on each parameter was also examined using linear regression analysis. This approach tests the linear change in the parameters across the drug doses (including no dose) without specifying pair-wise differences between dose levels.
All analyses were performed using the SAS System, Release 6.12 (SAS Institute Inc, Cary, North Carolina). Frequency distributions of the residuals from each model, as well as plots of the residuals by predicted value, were prepared and examined for normality and homogeneity of variance.
Results
Observations
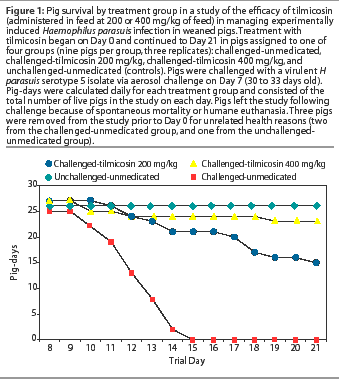
As demonstrated in Figure 1, mortality (death and humane euthanasia) among challenged-unmedicated pigs was relatively rapid, with death or humane euthanasia of all animals by Day 15 (8 days post challenge). Among challenged-medicated groups, mortality occurred gradually throughout the study period. Three pigs were removed from the study prior to Day 0 for unrelated health reasons (two from the challenged-unmedicated group, and one from the unchallenged-unmedicated group).
Among the challenged groups, both the 200 and 400 mg per kg tilmicosin dose level groups had lower mean clinical scores and higher mean final body weights, average daily gains, and mean total feed intakes, compared to the unmedicated group (P<.05). Results of pair-wise comparisons between treatment room-level averages of clinical and growth parameters are shown in Table 3. For the challenged-unmedicated group, the average maximum clinical score was 8.5, and 25 of 25 pigs (100%) developed clinical signs. For the challenged-tilmicosin 200 mg per kg group, the average maximum clinical score was 3.9, and 13 of 27 pigs (48%) developed clinical signs. For the challenged-tilmicosin 400 mg per kg group, the average maximum clinical score was 1.3, and 4 of 27 pigs (15%) developed clinical signs. Among the 39 animals that were euthanized or found dead between Day 0 and Day 21, the average maximum clinical score was 8.7.
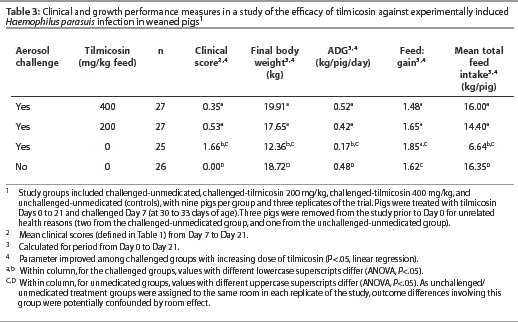
By linear regression analysis, mean clinical score and feed:gain declined (P<.05), and mean final body weight, average daily gain, and mean total feed intake rose (P<.05) with increasing dose of tilmicosin administered (Table 3). Among unmedicated groups, those challenged with H parasuis had higher mean clinical scores (P<.05) and lower mean final body weights, average daily gains, and mean total feed intakes (P<.05), compared to the unchallenged groups.
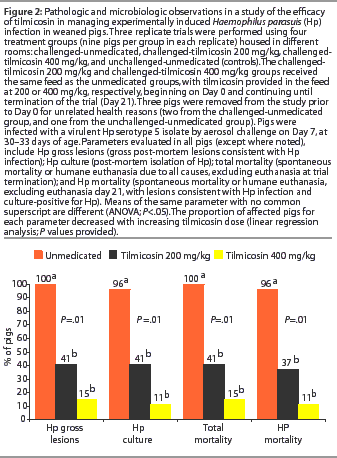
Among challenged groups, overall mortality and H parasuis-specific mortality were lower (P<.05) among those receiving 200 mg per kg and 400 mg per kg tilmicosin compared to the unmedicated group (Figure 2). By linear regression analysis, overall mortality and H parasuis-specific mortality declined (P<.05) with increasing dose of tilmicosin administered. Mortality did not occur in the unchallenged animals.
Necropsy
Among challenged groups, the proportions of pigs with gross lesions consistent with H parasuis infection, and with H parasuis-positive culture, were lower (P<.05) among those receiving 200 mg per kg or 400 mg per kg tilmicosin compared to the unmedicated group (Figure 2). By linear regression analysis, the proportion of animals positive for each of these parameters declined with increasing dose of tilmicosin administered (P<.05). No unchallenged pigs were positive for any of these parameters.
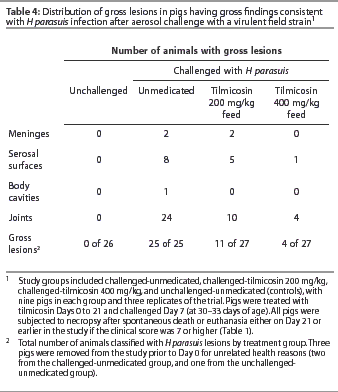
At necropsy, the number of animals with joint and serosal surface lesions declined with increasing tilmicosin dose (Table 4). Twenty-four of 25 challenged-unmedicated pigs (96%) demonstrated abnormal synovial fluid in joint spaces, compared to 10 of 27 challenged-tilmicosin 200 mg per kg pigs (37%) and 4 of 27 challenged-tilmicosin 400 mg per kg pigs (15%). All of the 16 H parasuis isolates randomly selected for serotyping were serotype 5.
Discussion
The results of this investigation demonstrate the effectiveness of prophylactic tilmicosin against experimentally induced H parasuis infection in swine. Improvements in all clinical and growth measures and all pathologic and microbiologic parameters were experienced with increasing dose of oral tilmicosin among challenged animals. With the exception of one of the 10 parameters examined (feed:gain), all outcomes were significantly improved (P<.05) in pigs receiving 200 and 400 mg tilmicosin per kg feed, compared to unmedicated animals. Overall trends in outcome improvement among the three treatment groups (challenged-unmedicated, challenged-tilmicosin 200 mg per kg, and challenged-tilmicosin 400 mg per kg) with increasing tilmicosin dose for all study parameters were significant (P<.05) by linear regression. The clinical improvements observed in this study are consistent with previously reported in vitro activity of tilmicosin against field strains of H parasuis.7
This study used an H parasuis challenge model that mimicked the natural aerosol route of pathogen exposure infecting high-health-status pigs. As unchallenged-unmedicated treatment groups were assigned to the same room throughout the study, outcome differences involving this group were potentially confounded by room effect. Nevertheless, among unmedicated groups, those challenged with H parasuis had higher mean clinical scores (P<.05) and lower mean final body weights, average daily gains, and mean total feed intakes (P<.05) compared to those unchallenged. Prior to this study, challenge systems involving high concentrations of organisms given intratracheally to specific-pathogen-free or caesarean-derived, colostrum-deprived pigs were required to produce characteristic H parasuis lesions.11,12
The appropriate use of antimicrobials is an important component of the management of H parasuis infection.2 Significant antigenic heterogeneity, both among and within the 15 currently identified H parasuis serovars, may potentially reduce the efficacy of vaccine programs.1,3,13-15 Protective immunogens may differ among strains, and several different strains may be present in a herd or even within the same animal at any one time.1,14,15 As vaccination does not always ensure effective herd immunity, not only is antimicrobial use indicated to treat clinical disease, but in addition, pre-exposure medication programs may also be of benefit. Medication before and after the manipulation or mixing of swine has been found to be of value.2 In the present investigation, the demonstrated benefits of oral tilmicosin provided pre-challenge support the latter observation.
Several characteristics are believed to contribute to the clinical effectiveness of tilmicosin. Tilmicosin has the unique ability to concentrate in, and be retained within, swine phagocytes.16 As neutrophils and macrophages migrate preferentially to sites of infection, this may provide a mechanism for achieving higher levels of antibiotic directly in the tissues where it is required. The release of bioactive tilmicosin by neutrophils after arriving at the site of infection has been demonstrated in vitro.16 Furthermore, tilmicosin stimulates lysosomal enzyme production and inhibits bacterial growth below minimum inhibitory concentration levels.16,17 At the time of writing, tilmicosin was not registered for the control of H parasuis infection in swine in the United States and Canada.
To our knowledge, this is the first study to demonstrate the in vivo effectiveness of an antimicrobial in the control of experimentally induced H parasuis infection. The results obtained provide evidence that tilmicosin administered in the feed is effective in the control of H parasuis infections in pigs.
Implications
- Tilmicosin administration beginning 7 days prior to aerosol challenge with a virulent H parasuis field strain was effective in controlling illness in weaned pigs.
- Improvements in clinical, growth, pathologic, and microbiologic parameters were experienced among challenged animals with increasing in-feed dose (200 and 400 mg per kg) of tilmicosin.
- At the time of writing, tilmicosin was not registered for the control of H parasuis infection in swine in the United States and Canada.
Acknowledgements
This study was supported by a grant in aid of research from Elanco Animal Health and by grants from the Ontario Ministry of Agriculture Food and Rural Affairs and the Natural Sciences and Engineering Research Council of Canada.
References – refereed
1. Done SH. Haemophilus parasuis: A synopsis. Pig J. 1999;44:207-221.
2. MacInnes JI, Desrosiers R. Agents of the "Suis-ide Diseases" of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can J Vet Res. 1999;63:83-89.
3. Rapp-Gabrielson VJ. Haemophilus parasuis. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:475-481.
5. Solano GI, Pijoan C, Rapp-Gabrielson V, Collins J, Carvalho LF, Winkelman N. Protective role of maternal antibodies against Haemophilus parasuis infection. Am J Vet Res. 1999;60:81-87.
7. DeRosa DC, Veenhuizen MF, Bade DJ, Shryock TR. In vitro susceptibility of porcine respiratory pathogens to tilmicosin. J Vet Diagn Invest. 2000;12:541-546.
8. Moore GM, Mowrey DH, Tonkinson LV, Lechtenberg KF, Schneider JH. Efficacy dose determination study of tilmicosin phosphate in feed for control of pneumonia caused by Actinobacillus pleuropneumoniae in swine. Am J Vet Res. 1996;57:220-223.
9. Moore GM, Basson RP, Tonkinson LV. Clinical field trials with tilmicosin phosphate in feed for the control of naturally acquired pneumonia caused by Actinobacillus pleuropneumoniae and Pasteurella multocida in swine. Am J Vet Res. 1996;57:224-228.
10. Olson LB, Backstrom LR. The effect of tilmicosin in minimizing atrophic rhinitis, pneumonia, and pleuritis in swine. Swine Health Prod. 2000;8:263-268.
11. Vahle JL, Haynes JS, Andrews JJ. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriologic, and morphologic findings. J Vet Diagn Invest. 1995;7:476-480.
12. Amano H, Shibata M, Kajio N, Morozumi T. Pathologic observation of pigs intranasally inoculated with Serovar 1, 4, and 5 of Haemophilus parasuis using immunoperoxidase method. J Vet Med Sci. 1994;56:639-644.
13. Rapp-Gabrielson VJ, Kocur GJ, Clark JT, Muir SK. Haemophilus parasuis: Immunity in swine after vaccination. Vet Med. 1997;92:83-90.
14. Rapp-Gabrielson VJ, Gabrielson DA. Prevalence of Haemophilus parasuis serovars among isolates from swine. J Vet Res. 1992;53:659-664.
15. Smart NL, Miniats OP, MacInnes JI. Analysis of Haemophilus parasuis isolates from southern Ontario swine by restriction endonuclease fingerprinting. Can J Vet Res. 1988;52:319-324.
16. Scorneaux B, Shryock TR. Intracellular accumulation, subcellular distribution and efflux of tilmicosin in swine phagocytes. J Vet Pharmacol Therap. 1998;21:257-268.
17. Diarra MS, Malouin F, Jacques M. Postantibiotic and physiological effects of tilmicosin, tylosin, and apramycin at subminimal and suprainhibitory concentrations on some swine and bovine respiratory tract pathogens. Int J Antimicrob Agents. 1999;12:229-237.
References – non refereed
4. Desrosiers R, Phaneuf JB, Broes A. An outbreak of atypical Glasser’s disease in Quebec. Proc IPVS. 1986;9:227.
6. Menard J, Moore C. Epidemiology and management of Glasser’s disease in SPF herds. Proc AASP. Denver, Colorado; 1990:187-200.