Original research | Peer reviewed |
Effect of Mycoplasma hyopneumoniae sow vaccination on piglet colonization at weaning
Alvaro R. Ruiz, DVM, PhD; Vitelio Utrera, DVM, PhD; Carlos Pijoan, DVM, PhD
Department of Clinical and Population Sciences, College of Veterinary Medicine, University of Minnesota, St Paul, MN 55108. Corresponding author: Dr C. Pijoan, Department of Clinical and Population Sciences, College of Veterinary Medicine, University of Minnesota, 385 An Sc/Vet Med Bldg, 1988 Fitch Avenue, St Paul, Minnesota 55108; Tel: 612-625-1233; Fax: 612-625-1210; E-mail: pijoa001@tc.umn.edu. ARR present address: Departamento de Patología y Medicina Preventiva, Facultad de Medicina Veterinaria, Universidad de Concepción, Casilla 537, Chillan, Chile.
Cite as: Ruiz AR, Utrera V, Pijoan C. Effect of Mycoplasma hyopneumoniae sow vaccination on piglet colonization at weaning. J Swine Health Prod 2003;11(3):131-135. Also available as a PDF.
Summary
Objective: To determine the prevalence of Mycoplasma hyopneumoniae at weaning in litters originated from vaccinated and non-vaccinated sows.
Materials and methods: A weekly production group of sows from an 800-sow commercial herd was randomly divided into two treatment groups. One group received a commercial vaccine (Respisure; Pfizer, New York) 5 and 3 weeks prior to farrowing, and the other group was unvaccinated (controls). Blood samples were collected from each sow prior to vaccination and at farrowing. Additionally, blood samples were collected at weaning and 2 weeks post weaning from four piglets from each sow. Samples were tested for antibodies to Mycoplasma hyopneumoniae by Tween-20 ELISA. Nasal swabs collected at farrowing from each sow and at weaning from each piglet were tested for M hyopneumoniae by nested polymerase chain reaction (N-PCR). The herd was not vaccinated for M hyopneumoniae before the study began, and no antibiotics effective against M hyopneu-moniae were used during the study. Two replicates were performed using sows and litters in 2 consecutive weeks of production.
Results: Over 80% of the vaccinated sows seroconverted and their antibodies were transferred to the piglets via colostrum. Prevalence of M hyopneumoniae in nasal swabs from sows did not differ among groups. Prevalence in piglets was lower in litters from vaccinated sows, but was significantly lower only in the first replicate.
Implications: Vaccination of sows against M hyopneumoniae reduced prevalence of N-PCR-positive piglets at weaning, and could be used to control M hyopneumoniae in modern swine production systems.
Keywords: Mycoplasma hyopneumoniae, vaccine, prevalence
Search the AASV web site for pages with similar keywords.
Mycoplasma hyopneumoniae is a primary agent associated with enzootic pneumonia and the porcine respiratory disease complex (PRDC), which are considered the most important respiratory syndromes in modern swine production.1 Both syndromes are distributed worldwide and have an important economic impact in the industry.
In recent years, a number of efficacious vaccines against M hyopneumoniae have been introduced and widely accepted by the industry. Both protective immunity and less severe lesions (and presumably less severe disease) have been demonstrated in animals vaccinated against M hyopneumoniae. Under experimental challenge, active immunity induced by the vaccine also slightly reduced colonization.2
One recommended protocol is to vaccinate suckling piglets at about 7 days of age, with a booster vaccination at 21 days, usually at weaning. The rationale for this protocol is based on the traditional epidemiology of enzootic pneumonia, which is characterized by coughing signs occurring at the end of the nursery period and the beginning of the finishing period (8 to 12 weeks of age).3 This early presentation of the disease, suggesting that infection takes place 4 to 6 weeks earlier, is characteristic of one-site continuous flow herds. Because there is a potential problem with maternal antibodies interfering with the response of the piglets to vaccination,4 vaccination of both sows and their piglets has been questioned.
As the swine industry has moved into high health systems through the use of segregated production, the epidemiology of the disease has changed. The emergence of PRDC, a late-finishing clinical pneumonia for which M hyopneumonia is a primary agent, demonstrates that the traditional model of early nursery disease no longer holds true for the many modern swine herds. Unlike traditional nursery mycoplasma pneumonia, PRDC is not always controlled by piglet vaccination, requiring a return to mass usage of feed grade antibiotics, even in high health segregated early weaning herds.
Prevalence at weaning is an indicator of the number of sows in the farrowing group that are shedding M hyopneumoniae, and of their immunity level. In the study of Calsamiglia et al,5 using polymerase chain reaction- (PCR-) based diagnostics, the prevalence of M hyopneumoniae infection in sows, even at later parities, was much greater than previously thought. However, these infected sows rarely infect their piglets prior to weaning.6 We believe that this discrepancy is a reflection of high maternal immunity that protects most litters from infection, even in the presence of microbial shedding by the sow. Therefore, a group of sows with a high level of immunity produces groups of weaned piglets with very low prevalence of infection. Detection of M hyopneumoniae colonization is very difficult using traditional bacteriological methods. The recent advent of nested-PCR (N-PCR) technology allows for detection of the antigen in nasal swabs obtained from live animals. This technique can therefore be used to estimate colonization prevalence in a pig population.7
The purpose of this study was to determine the prevalence of M hyopneumoniae in nasal swabs collected at weaning from litters that originated from vaccinated and non-vaccinated sows in a commercial herd that had a weekly production system and that did not vaccinate against M hyopneumoniae.
Materials and methods
Herd
The experiment was performed in an 800-sow commercial herd located in the central part of Minnesota during the fall of 2001. Piglets were weaned at 19 days of age. The herd did not use M hyopneumoniae vaccine nor feed or water medication effective against mycoplasma. Prior to the beginning of the experiment, coughing was detected in finishing pigs, and approximately 20% of piglets were positive for M hyopneumoniae by N-PCR before weaning.
Experimental design
A weekly farrowing group of sows was divided into two treatment groups housed in different rooms. Sows were selected to provide an even distribution of parities in each study group. Group 1 (V group) was constituted of sows vaccinated twice, 5 and 3 weeks before farrowing (Respisure; Pfizer, New York), and their non-vaccinated piglets. Group 2 (NV group) were unvaccinated controls. Two replicates of the study were performed using two consecutive weekly farrowing groups, with 38 sows in the first replicate (19 sows per treatment group) and 36 sows in the second replicate (18 sows per treatment group). Sows from all parities were represented in each group. Four piglets per sow were randomly selected to be tested for M hyopneumoniae serologically and by N-PCR, with a total of 152 piglets in the first replicate and 144 in the second replicate.
Parity distribution was kept as similar as possible between treatment groups in order to minimize confounding factors that might influence the study. Cross fostering was performed only within treatment groups, and was limited to situations in which sows would be unable to raise their whole litter. Cross fostering was also limited to the first 12 hours after birth, in order to strictly minimize colonization of the piglets from their original sows.6
Parameters measured
For each group and each replicate, four parameters were measured.
Sow antibody titers prior to vaccination and at farrowing. Blood samples were collected the same day for both treatment groups in each replicate. The first samples were collected the day that the first vaccination was administered. Samples were collected from the V group before the sows were vaccinated. The second samples were collected in the middle of the week of farrowing (Wednesday).
Piglet antibody titers at weaning and at 2 weeks post weaning. Blood samples were collected the day of weaning and 2 weeks later for both treatment groups in each replicate.
Prevalence of nasal N-PCR-positive sows at farrowing. Nasal swabs were collected from each sow in the middle of the week of farrowing (Wednesday).
Prevalence of nasal N-PCR-positive pigs at weaning. Nasal swabs were collected the day of weaning from four piglets per sow in each treatment group in each replicate.
Serology
Seroconversion to M hyopneumoniae was evaluated by a Tween-20 ELISA, considering a sample-to-positive (S:P) value of 1.0 as a positive result.8
Nested PCR
Nested PCR was performed as described previously.9,10 This technique has been routinely performed in our laboratory for all M hyopneumoniae-suspect submissions to the Minnesota Diagnostic Laboratory (St Paul, Minnesota).
Statistical analysis
Nested PCR results from nasal swabs were analyzed using two-by-two tables, with positive or negative results as a cut-off point. ELISA results were analyzed by ANOVA with a 95% confidence interval (P<.05). The numbers of positive animals per group and the numbers of sows per parity category were compared by a chi-square distribution. Mean S:P ratios of the piglets in each treatment group were compared at the two data points by repeated measures ANOVA.
Results
Parity distribution
As a result of the distribution of sows’ individual parities, animals were classified according to their parity in three categories: 0-1, 2-4, and 5 or greater (5+). Category 0 comprised gilts that had not farrowed a litter.
Parity distribution between treatment groups was similar but not identical (Table 1). In the first replicate, there were more animals in parities 2-4 and fewer in parities 5+ among the non-vaccinated sows (NV group) than among the vaccinated sows (V group). However, there was no statistical difference in parity distribution in either the first or second replicate (P>.05).
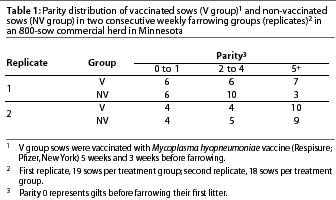
Serological results
First replicate. The means of the ELISA S:P ratios of the first replicate are shown in Figure 1A. Two sows in the V group and two sows in the NV group were seropositive to M hyopneumoniae prior to vaccination. One sow (NV group) became seronegative at farrowing. These four seropositive animals had all received a M hyopneumoniae vaccine before arrival in the herd. These were the only gilts included in this replicate, and the only animals in the study, that were vaccinated for M hyopneumoniae prior to the beginning of the study.
Ninety-four percent of the V group sows (18 of 19) were seropositive at farrowing, 81% of their piglets (62 of 76) were sero-positive at weaning age, and 75% of the piglets (57 of 76) remained seropositive at 2 weeks post weaning. On the other hand, only 5% of the NV group piglets (4 of 76) were seropositive at weaning age and 4% (3 of 76) remained seropositive 2 weeks later.
The mean ELISA S:P ratios prior to vaccination did not differ between treatment groups (Figure 1A). However, mean S:P ratios were higher in the V group than in the NV group at farrowing, and this difference was also observed in the piglet groups that originated from these sows.
The S:P ratios in the V group (sows and their piglets) showed a normal distribution, with the mean S:P ratio lower (P<.01) for the sows prior to vaccination than for the same sows at farrowing, and higher for the piglets of these sows both at weaning and 2 weeks post weaning compared to the sows prior to vaccination (P<.01). The mean S:P ratios for the V group piglets at weaning and 2 weeks post weaning did not differ (P>.05).
Second replicate. The means of the ELISA S:P ratios of the second replicate are shown in Figure 1B. Four sows were seropositive to M hyopneumoniae prior to vaccination. One was a gilt in the V group (the only gilt in this replicate), and the other three (parities 3, 4, and 10) were in the NV group. In later samples from these three sows, one became negative and one suspicious, and the other remained positive.
Eighty-three percent of the V group sows (15 of 18) seroconverted at farrowing, 82% of their piglets (59 of 72) were seropositive at weaning age, and 70% of the piglets (51 of 72) remained positive at 2 weeks post weaning. Three piglets were seropositive at weaning in litters from seronegative V group sows, and one of these became seronegative at 2 weeks post weaning.
On the other hand, only 21% of the piglets (15 of 72) of the NV group sows were seropositive at weaning age, and 15% (11 of 72) remained seropositive 2 weeks later. Six of these were offspring of two of the three seropositive NV sows.
There were no differences in mean S:P ratios between treatment groups prior to vaccination (Figure 1B). However, mean S:P ratios were higher in the V group than in the NV group after vaccination, and this difference was also observed in the piglet groups that originated from these sows.
The S:P ratios in the V group (sows and their piglets) showed a normal distribution, with the mean S:P ratio lower (P<.01) for the sows prior to vaccination than for the same sows at farrowing, and higher (P<.01) for the piglets of these sows both at weaning and 2 weeks post weaning compared to the sows prior vaccination. The mean S:P ratio was lower (P<.01) for the V group piglets 2 weeks post weaning than for the same piglets at weaning.
Nested-PCR results
No relationship was observed between parity and N-PCR positive results in the sows in either replicate (data not shown).
First replicate. Eighteen percent of the sows (7 of 38) were positive for M hyopneumoniae by N-PCR, with no statistical differences between treatment groups (P=.4). There were more N-PCR-positive piglets in the NV group than in the V group (Table 2). Only two piglets of 76 (2.6%) were positive by N-PCR in the V group, one from an N-PCR-positive sow and the other from a negative sow (Table 2). Ten piglets of 76 (13.2%) were positive by N-PCR in the NV group. Seven of the ten N-PCR-positive piglets were from N-PCR-negative sows (Table 2). All of the N-PCR-positive piglets, as well as their dams, were seronegative.
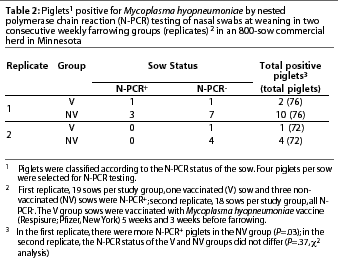
Second replicate. No sows in either treatment group were positive for M hyopneumoniae by N-PCR. Nasal N-PCR prevalence in piglets at weaning did not differ between treatment groups (Table 2). Only one piglet of 72 (1.4%) was positive by N-PCR in the V group (Table 2), and this animal was also seropositive. Four piglets of 72 (5.5%) were positive by N-PCR in the NV group (Table 2), and three of them were seropositive.
Discussion
The objective of this study was to determine if vaccination of sows modulated the prevalence of M hyopneumoniae in nasal swabs from their offspring at weaning. In order to minimize the effect of sow parity, parity distribution among groups was kept similar, and no significant differences were observed among sow parity categories in either replicate. Sow parity, as well as sow immune status, may have an effect on M hyopneumoniae shedding or in colonization of the offspring, but this experiment did not address this issue. In order to answer these questions, further research with a larger number of different parity sows will be needed.
More than 80% of the V group sows seroconverted following vaccination, which was expected with the commercial vaccine used. This result is in agreement with other studies that have shown some variation in seroconversion following vaccination, ranging from 30 to 100%.2,10 Introduction of vaccinated gilts into the herd may explain why some sows were seropositive prior to vaccination. The presence of these sero-positive sows probably also indicates that the microorganism was present in the herd, a fact which was confirmed by N-PCR before the study began.
Maternal antibodies were transferred to the piglets via colostrum, and it was possible to observe the decay of these antibodies between the two sample times, which was statistically significant in the second replicate. The transfer of passive immunity to the piglets via colostrum, and its decay, are well documented for M hyopneumoniae.11,12 Most of the immunoglobulin present in colostrum originates from serum.11 The mean ELISA S:P ratio of the vaccinated sows at farrowing was significantly higher than that of the offspring in both replicates at both sample times. This may be explained by the rapid decay of M hyopneumoniae maternal antibodies in the piglets (median half-life, 15.8 days).12 The presence of a few seropositive piglets in litters from seronegative sows may be explained by the limited cross fostering that took place, in situations where piglets were transferred after they had suckled colostrum. However, the exact information about which piglets were moved was not available.
There were also a few N-PCR-positive piglets in litters derived from N-PCR-negative sows. These may be explained in several ways. The N-PCR test may not have been sensitive enough to detect the presence of the organism in some sows, although as few as 102 M hyopneumoniae organisms may be detected by the N-PCR. Alternatively, sows may shed the organism intermittently, which might explain why some apparently negative sows had positive piglets at weaning. Mycoplasma hyopneumoniae is not a normal inhabitant of the nose, and it is believed that the microorganism is present in that site either in the early stages of the infection (presumably consisting of dead or non-attaching M hyopneumoniae organisms), or when the animal is actively shedding the microorganism.13 Because of this, a single sampling in a herd provides a picture of herd prevalence, but not of the real status of a particular animal. We have shown that N-PCR-negative animals may be colonized by M hyopneumoniae.13 Lastly, some of the paradoxical N-PCR results may have been the outcome of the limited cross fostering that took place. However, it is doubtful that cross fostering affected the N-PCR results of this study, since it was limited to the first 12 hours after birth and was performed only within treatment groups. Piglet serology results corroborated that cross fostering had indeed been limited to treatment groups.
There were no significant differences in sow N-PCR results between treatment groups. In the second replicate, all sows were N-PCR-negative at farrowing. Sows were sampled at farrowing because it was thought that during this stressful time, shedding of M hyopneumoniae might be maximized. However, there is little scientific evidence for this, and more research will be needed in order to identify the time of maximal shedding. No relationship was observed between parity and N-PCR positive results in the sows, which is in agreement with previous research.5
The number of N-PCR-positive piglets at weaning was higher in the NV group than in the V group. Although this difference was statistically significant only in the first replicate, a trend was also observed in the second replicate. Since we could not detect positive sows by N-PCR in the single sampling of the second replicate, the prevalence of M hyopneumoniae must have been much lower in this group than in the first. In off-site production systems, there are considerable differences in disease incidence among production groups. Diseases that develop within these groups probably reflect colonization before weaning, which originates from the sows. This relationship between sow and piglet prevalence was documented in this study.
It is not clear if the lower prevalence of N-PCR-positive piglets in the V group, compared to prevalence in the NV group, occurred because the sows were shedding lower levels of microorganisms, or because passive immunity from the vaccinated sows protected the V group piglets from becoming N-PCR-positive. Since two of the three N-PCR-positive V group piglets were seronegative, this suggests a role of antibodies in preventing colonization.
In order to minimize the effect of variables such as seasonal effect, management differences, and differences between sow groups, two weekly production groups were divided into the two treatment groups, each having similar parity distribution and under the same management. Additionally, the experiment was done in consecutive production weeks in an effort to reduce or eliminate the seasonal effect. However, this approach prevented us from obtaining information about seasonal variation, and from learning whether the results are representative across different herds. However, these results suggest that vaccination of sows reduced the prevalence of M hyopneumoniae in their pigs at weaning.
Further research is required to confirm that sow vaccination against M hyopneumoniae reduces the prevalence of N-PCR-positive piglets and to evaluate the effect of this difference on production parameters during the nursery and finishing periods.
Implications
- Under the conditions of this study, sow vaccination against M hyopneumo-niae reduced the prevalence of N-PCR-positive piglets at weaning.
- Sow vaccination could be used to control M hyopneumoniae in production systems that practice strict all in-all out management and offsite weaning.
Acknowledgements
The authors thank Dr Allan Carlson for his cooperation in finding the herd and helping with these studies. This project was partly supported by grants from the National Pork Producers Association and from Pfizer Animal Health.
References – refereed
1. Ross R. Mycoplasmal diseases. In: Straw B, D’Allaire S, Mengeling W, Taylor D, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:495-510.
2. Thacker E, Thacker B, Boettcher T, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. Swine Health Prod. 1998;6:107-112.
3. Taylor DJ. Mycoplasma infections. In: Taylor DJ, ed. Pig Diseases. 7th ed. Cambridge: St Edmundsbury Press Ltd; 1999:180-190.
5. Calsamiglia M, Pijoan C, Bosch G. Profiling Mycoplasma hyopneumoniae in farms using serology and a nested PCR technique. Swine Health Prod. 1999;7:263-268.
6. Calsamiglia M, Pijoan C. Colonisation state and colostral immunity to Mycoplasma hyopneumoniae of different parity sows. Vet Rec. 2000;146:530-532.
7. Ruiz A, Galina L, Pijoan C. Mycoplasma hyopneumoniae colonization in pigs sired by different boars. Can J Vet Res. 2002;66:79-85.
8. Nicolet J, Paroz P, Bruggmann S. Tween 20 soluble proteins of Mycoplasma hyopneumoniae as antigen for an enzyme linked immunosorbent assay. Res Vet Sci. 1980;29:305-309.
9. Calsamiglia M, Pijoan C, Trigo A. Application of a nested polymerase chain reaction assay to detect Mycoplasma hyopneumoniae from nasal swabs. J Vet Diagn Invest. 1999;11:246-251.
10. Mattsson JG, Bergstrom K, Wallgren P, Johansson KE. Detection of Mycoplasma hyopneumoniae in nose swabs from pigs by in vitro amplification of the 16S rRNA gene. J Clin Microbiol. 1995;33:893-897.
12. Bourne F, Curtis J. The transfer of immuno-globins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology. 1973;24:157-162.
13. Morris CR, Gardner I, Hietaba S, Carpenter T, Anderson R, Parker K. Persistence of passively acquired antibodies to Mycoplasma hyopneumoniae in a swine herd. Prev Vet Med. 1994;2:29-41.
References – non refereed
4. Thacker B, Thacker E. Influence of maternally-derived antibodies on the efficacy of a Mycoplasma hyopneumoniae bacterin. Proc AASV. Nashville, Tennessee. 2001:513-515.
11. Thacker E. Current status of Mycoplasma vaccination. Proc Swine Dis Conf Swine Pract. Ames, Iowa. 2001:69-71.