Original research |
Peer reviewed |
Efficacy of ceftiofur sodium for the control of mortality in neonatal pigs orally inoculated with K88+ (F4+) enterotoxigenic Escherichia coli
Beth Hibbard, MA; Fabian M. Kausche, Dr med vet, MS; Kelly F. Lechtenberg, DVM, PhD; Michael S. Hanna, DVM;
Edward J. Robb, DVM, MS; W. Lawrence Bryson, PhD; S. Theodore Chester, Jr, PhD; Kenneth J. Dame
BH, FMK, EJR, WLB, STC, KJD: Pharmacia Animal Health, Kalamazoo, Michigan; KFL, MSH: Midwest Veterinary Services, Oakland, Nebraska. Corresponding author: Beth Hibbard, Pharmacia Animal Health, 7000 Portage Rd, Kalamazoo, MI 49001; Tel: 269-833-2630; Fax: 269-833-3246; E-mail: beth.hibbard@pharmacia.com.
Hibbard B, Kausche FM, Lechtenberg KF, et al. Efficacy of ceftiofur sodium for the control of mortality in neonatal pigs orally inoculated with K88+ (F4+) enterotoxigenic Escherichia coli.J Swine Health Prod. 2003;11(1):7-11. Also available as a PDF.
Summary
Objective: To evaluate the efficacy of ceftiofur sodium (Naxel/Excenel Sterile Powder; Pharmacia & Upjohn, Kalamazoo, Michigan) administered IM once daily at 3 or 5 mg ceftiofur per kg BW for 3 days for control of mortality associated with K88+ (F4+) Escherichia coli-induced colibacillosis in neonatal piglets.
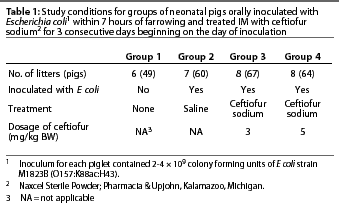
Methods: Pregnant gilts susceptible to colibacillosis associated with K88+ E coli were farrowed in farrowing crates with solid walls to prevent contact between litters. A total of 29 gilts with 240 piglets were enrolled, with litters randomly assigned to study group by order of farrowing. Groups included uninoculated-no treatment, inoculated-sterile saline IM, inoculated-ceftiofur sodium IM (3 mg per kg), and inoculated-ceftiofur sodium IM (5 mg per kg). Treatments were administered on Days 0, 1, and 2. On Day 0, each inoculated piglet received 20 mL inoculum orally, containing approximately 108 colony forming units per mL of E coli strain M1823B (O157:K88ac:H43). Clinical observations were conducted twice daily for 8 days.
Results: Mean mortality rates for the two ceftiofur-treated groups were lower than for the inoculated-saline group (P=.003). The proportion of normal stool scores was higher in ceftiofur-treated groups than in the inoculated-saline group (P<=.008). Average daily gain for surviving piglets did not differ among the groups.
Implications: Ceftiofur sodium administered IM daily for 3 consecutive days at 3 or 5 mg per kg significantly reduced mortality and increased percentage normal stool scores in treated piglets inoculated with K88+ E coli, compared to untreated, inoculated piglets.
Keywords: swine, bacteria, Escherichia coli, enterotoxins, diarrhea
Received: August 22, 2001
Accepted: December 14, 2001
Diarrhea occurring in piglets within the first few days after birth (0 to 4 days) is often caused by Escherichia coli infection of the jejunum and ileum.1 The disease is economically important in the swine industry due to the losses observed in the intensive management systems currently used in farrowing houses.1 Causative bacteria adhere to the epithelium of the small intestine by means of fimbrial adhesins and produce one or more enterotoxins that may increase fluid secretion in the small intestine up to tenfold, causing diarrhea if excess fluid is not absorbed in the large intestine.1 No primary inflammatory reactions occur in this type of hypersecretory neonatal diarrhea.1 However, the ability of the large intestine to compensate for sudden fluid flux is easily overwhelmed in neonates.1
Antibiotic therapy is indicated for the treatment of neonatal diarrhea caused by enterotoxigenic E coli, such as K88+ (F4+) strains.1 Therapy should be rapidly instituted before piglets become severely dehydrated and should seek to inhibit growth of enterotoxigenic bacteria in the small intestine. Fluid and electrolyte replacement is useful in treating dehydration and acidosis. Antibacterial treatment should be coupled with appropriate husbandry techniques, which include providing a draft-free, warm environment, using farrowing crates designed to keep the piglets away from fecal material, and maintaining appropriate sanitation of the sow and the farrowing facility.1
Ceftiofur sodium (Naxcel/Excenel Sterile Powder; Pharmacia & Upjohn, Kalamazoo, Michigan) is currently approved in the United States and many other countries for the control and treatment of swine respiratory disease (SRD), administered IM at 3 or 5 mg ceftiofur per kg BW for 3 consecutive days. However, its spectrum of activity and pharmacokinetic and pharmacodynamic characteristics, including tissue homogenate data, support a clinical rationale for use of ceftiofur sodium in the treatment of enterotoxigenic colibacillosis in neonatal piglets. Ceftiofur was confirmed to be an effective treatment for colibacillosis in an enterotoxigenic K99+ E coli challenge model study2 and in field studies where oral3 and IM4 administration were evaluated for the treatment of naturally occurring colibacillosis. The present study evaluated the efficacy of ceftiofur sodium administered IM for 3 consecutive days at 3 or 5 mg ceftiofur per kg BW for the control of mortality associated with secretory diarrhea in neonatal piglets orally inoculated with K88+ E coli.
Materials and methods
Experimental design
Gilts were obtained and placed in standard farrowing crates at least 14 days before their expected farrowing date. Litters (n=29) were randomly assigned in order of farrowing to one of four study groups (Table 1). Birth weights were obtained when piglets were processed at the end of farrowing. Piglets in Groups 2, 3, and 4 were inoculated within 7 hours of the end of farrowing (Day 0). Clinical signs were observed twice daily, beginning 12 hours after the end of farrowing, and through one observation on Day 8. The first treatment was administered to all designated piglets 6 hours after inoculation on Day 0, regardless of clinical signs. The first clinical evaluation was made either before or after the first treatment, depending on the time of inoculation. Day 1 and Day 2 treatments were administered at 24-hour intervals. The personnel who conducted the clinical evaluations and necropsies remained blinded to the assigned treatments throughout the trial. The trial was conducted according to the Guidelines for Good Target Animal Study Practices5 and was consistent with applicable laws and regulations governing the humane care of animals.
Study animals
Pregnant Lancaster or Large White cross gilts (n=31) that had not been vaccinated against E coli were obtained from a source that produced piglets susceptible to enterotoxigenic diarrhea associated with K88+ E coli, with litter mortality rates between 0 and 60% (unpublished data). Two groups of gilts (n=11 and n=20) were placed in standard farrowing crates (with solid walls to prevent nose-to-nose contact between litters) to acclimatize for at least 14 days before their expected farrowing date. Gilts were observed every 2 to 4 hours to assist in farrowing as needed and to document farrowing times. Cross-fostering was allowed before inoculation to balance litter size. All piglets with obvious physical problems, injuries, or birth weight <0.9 kg were excluded from the study, as was any piglet born after the litter had been inoculated.
During the entire acclimatization and study periods, the only antibacterials administered to the piglets were the experimental ceftiofur treatments included in the study design. No antibacterials were administered to the gilts during the acclimatization and study periods.
Administration of treatments
Ceftiofur sodium was diluted to 10 mg ceftiofur per mL for accurate dosing and administered IM using a 20-gauge, 2.5-cm sterile needle. The first treatment (Day 0) was administered in the left neck, and Day 1 and Day 2 treatments were administered in the right and left neck, respectively.
Inoculum
Each inoculated piglet received 20 mL of inoculum orally, containing approximately 108 colony forming units (CFUs) per mL of E coli strain M1823B (O157:K88ac:H43) suspended in trypticase soy broth. This isolate, a hemolytic strain originally isolated from the intestine of a pig with post-weaning diarrhea, produces the heat labile enterotoxin (LT) and the heat stable toxin (STb).6 The ceftiofur minimum inhibitory concentration (MIC) for this organism is 0.5 mg per mL. The inoculum was administered in 10-mL portions, with all piglets in a litter receiving the first 10 mL before any piglet received the second 10 mL. Inocula were administered orally with the aid of a 13-cm, 14-gauge catheter (Angiocath; BD Medical Systems, Franklin Lakes, New Jersey) with the stylet removed. A new catheter was used for each litter. Throughout the study, personnel changed gloves after handling a litter and before moving to the next, to minimize the possibility of spreading the challenge organism between litters. In addition, care was taken when cleaning crates to avoid splashing fecal material into adjacent crates.
Clinical observations and scoring
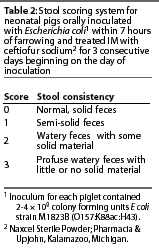 Observers recorded weights, determined a stool score using the scoring system
described in Table 2, and noted an illness index score and other relevant clinical
ob-servations.Clinical scoring was performed by evaluators who remained blinded to
the assigned study groups throughout the study.
Observers recorded weights, determined a stool score using the scoring system
described in Table 2, and noted an illness index score and other relevant clinical
ob-servations.Clinical scoring was performed by evaluators who remained blinded to
the assigned study groups throughout the study.
Moribund or savaged piglets were euth-anized. All piglets removed from the study or found dead were necropsied. Deaths were categorized as due to colibacillosis if evidence of dehydration was present at necropsy.
Mortality due to enterotoxigenic colibacillosis was calculated as the proportion of piglets in a litter that died due to dehydration associated with enterotoxi-genic colibacillosis. Percent normal stool scores was calculated for each surviving piglet as the number of normal stool scores divided by the number of stool evaluations for that piglet. Average daily gain was calculated for the surviving piglets as the final weight of each piglet minus the birth weight, divided by the number of days the piglet was on study.
No rectal swabs, fecal samples, or tissue samples were collected for culturing during this study.
Statistical analysis
Data from all piglets that died or were euthanized prior to inoculation, or that were born after the rest of the litter was inoculated, were excluded from the analysis data set. The primary variable in this randomized block design study was mortality associated with dehydration due to entero-toxigenic colibacillosis. Stool scores and ADG were secondary variables. Mortality was analyzed as a general linear mixed model using the PROC MIXED procedure from SAS.7 Mortality was expressed as the Freeman-Tukey angularly transformed proportion of piglets within a litter that died due to enterotoxigenic colibacillosis (using n + 0.5 as weights, where n = number of piglets assigned to the study within the litter). The mixed model included the fixed effects of period (farrowing period for the two groups of gilts), treatment, and period by treatment interaction, and the random effect of gilt-litter within period by treatment. The random effect of gilt-litter within period was used as the error term to test the fixed effects of treatment.
Stool scores for the surviving piglets were analyzed as a general linear mixed model using the PROC MIXED procedure from SAS. Stool scores were expressed as the Freeman-Tukey angularly transformed proportion of normal stool scores for each piglet (using n + 0.5 as weights, where n = number of stool evaluations). The mixed model included the fixed effects of period (farrowing period), treatment, and period by treatment interaction, and the random effects of gilt-litter within period by treatment and residual. The random effect of gilt-litter within period was used as the error term to test the fixed effects of treatment.
The ADG for surviving piglets was analyzed as a general linear mixed model using the PROC MIXED procedure from SAS. The mixed model included the fixed effects of period (farrowing period), treatment, and period by treatment interaction, and the random effects of gilt-litter within period by treatment and residual. The random effect of gilt-litter within period was used as the error term to test the fixed effects of treatment.
For each variable, pairwise comparisons were made between Groups 3 and 4 and Group 2. The farrowing period by treatment interaction for each comparison was accomplished through contrast statements. No statistical comparisons were made between Group 1 and the other study groups or between Groups 3 and 4. All analyses were conducted using [alpha]=.05.
Results
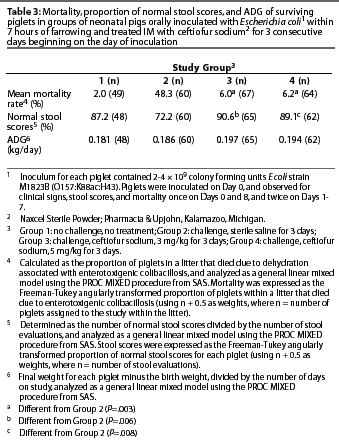
A total of 29 gilts with 240 piglets were included in the statistical analyses. Data from five piglets whose deaths were not related to colibacillosis were excluded before the analyses of stool scores and ADG.
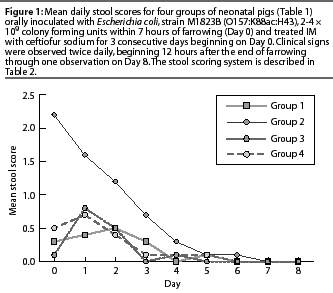
Results of analysis of mean mortality due to colibacillosis, proportion of normal stool scores in surviving piglets, and ADG in surviving piglets are presented in Table 3. Mean mortality rates for Groups 3 and 4 were lower than for Group 2, and the proportionof normal stool scores during the entire study period was higher in Groups 3 and 4 than in Group 2. Daily stool scores (which were not analyzed statistically) were numerically higher through Day 4 for Group 2 compared to Groups 3 and 4 (Figure 1). There were no significant differences in ADG between Group 2 and Groups 3 or 4 (P>.05).
Discussion
The results of this study provide clinical confirmation of the effectiveness of ceftiofur for the treatment of enterotoxi-genic colibacillosis in neonatal piglets. These data are consistent with the pharmacokinetics and pharmacodynamics of ceftiofur.8
Salmon et al9 determined ceftiofur MIC for 84 swine E coli isolates using the microdilution method of the National Committee on Clinical Laboratory Standards with appropriate quality control strains. The MIC50 (which represents the MIC for the most sensitive 50% of the isolates) was 0.5 mg per mL and the MIC90 (which represents the MIC for the most sensitive 90% of the isolates) was 1.0 mg per mL (range 0.25 to 4.0 mg per mL, mode 0.5 mg per mL). These values are comparable to the ceftiofur MICs for Salmonella serovar Choleraesuis, the least ceftiofur-sensitive pathogen associated with SRD for which ceftiofur sodium and ceftiofur hydrochloride have label claims.10,11 In addition, 885 swine enteric isolates, including E coli, collected in Missouri in 1996-1997, were susceptible to ceftiofur.12 The ceftiofur MIC for the enterotoxigenic E coli inoculation strain used in the present study was 0.5 mg per mL, in agreement with the mode and MIC50 of previously reported swine E coli isolates.9
In a pharmacokinetic study evaluating the plasma concentrations of ceftiofur sodium or ceftiofur hydrochloride in pigs,8 the critical pharmacokinetic parameters (maximum plasma concentration achieved after treatment administration, area under the plasma concentration-time curve, and time the plasma concentration remained above 0.2 mg per mL) were similar for pigs receiving either ceftiofur sodium or ceftiofur hydrochloride in the same dosage. The dosing regimens tested for the treatment of colibacillosis in our study included the range of dosing regimens approved by the Food and Drug Administration Center for Veterinary Medicine for the treatment and control of swine bacterial respiratory disease.
A tissue distribution study conducted in pigs provides further evidence supporting the effectiveness of ceftiofur for enteric colibacillosis.13 Pigs received a single IM dose of ceftiofur sodium at 3 mg ceftiofur per kg BW, the lower end of the dose range approved in the United States for the treatment of SRD. Ceftiofur concentrations in the washed homogenate of the distal 1 m of jejunum and in mesenteric lymph node were 1.00 and 1.09 mg per g, respectively, 7.5 hours after treatment.
Although a formal dose proportionality study evaluating the pharmacokinetics of ceftiofur when administered at several incremental dose levels has not been conducted in swine, previous studies support dose proportionality in swine.14 Assuming dose proportionality, the tissue concentrations in the distal portion of the jejunum would be expected to remain above the MIC90 for the E coli strains tested9 for approximately 18.5 to 19.5 hours after a single IM dose of 5 mg ceftiofur per kg BW, and tissue concentrations are estimated to be approximately 0.68 mg ceftiofur per mL at the 24-hour dosing interval. Since current technology cannot accurately measure the amount of ceftiofur that bacteria encounter at the site of colonization, namely, the luminal surface of the small intestine, it is not technically feasible to determine the ratio of the drug concentration at the site of infection to the MIC. While therapeutic levels of ceftiofur-related residues were detected in tissue samples of the small intestine13 when pigs were administered ceftiofur, rapid degradation of ceftiofur in the large intestine and fecal material of animals15,16 would likely limit its effectiveness against infections in the large intestine.
The results of this inoculation study confirm and extend previous work that evaluated the efficacy of ceftiofur for the treatment of colibacillosis. In a similar study conducted with a K99+ E coli, a single dose of ceftiofur hydrochloride at 0.5 or 2.0 mg ceftiofur per kg BW was administered IM to piglets 6 hours after inoculation.2 In pigs treated with either dose compared to untreated controls, mortality and abnormal stool scores were significantly lower, there was significantly less shedding of the challenge organism, and weight gain was significantly greater. Groups treated orally were also included in this study and a second challenge study.2 All oral treatment regimens reduced mortalityand abnormal stool scores and improvedweight gain relative to untreated controls. The efficacy of the oral treatments was later confirmed in two field studies.3,4 In a multilocation field efficacy study3 of ceftiofur for treatment of naturally occurring colibacillosis, a single oral dose of 13.5 mg ceftiofur per pig was effective, ie, reduced mortality and diarrhea in the surviving piglets compared with placebo-treated controls. The oral use of ceftiofur is no longer considered to be prudent use of a third generation cephalosporin. A second field study evaluated IM single-dose and 3-day regimens of 8 mg ceftiofur per kg (pigs <10 days old) and 11 mg ceftiofur per kg (pigs >=10 days old) compared to untreated controls.4 Clinical recovery rates and weight gains were higher for ceftiofur-treated groups. The efficacy demonstrated in these clinical studies supports the conclusion that ceftiofur is present at the mucosal surface of the small intestine at concentrations adequate for the treatment of colibacillosis in pigs.
While the present study provided clinical confirmation of the efficacy of ceftiofur for reduction of mortality associated with enterotoxigenic colibacillosis in neonatal swine, it is important to note that this is extra-label use of ceftiofur. A dose titration has not been performed for this use of ceftiofur, and the doses tested in the present study were those currently approved by the FDA and other regulatory bodies worldwide for the treatment and control of bacterial SRD. Although there are no systematic sampling studies currently conducted to evaluate trends in enterotoxigenic E coli antimicrobial resistance, data suggest that resistance may develop in some swine pathogens.17 This emphasizes the importance of having a definitive diagnosis and knowledge of the MIC(s) for the pathogens prior to initiation of antimicrobial treatment. These clinical data are provided for practitioner information only to assist in the decision-making process regarding treatment selection.
Implications
- Under the conditions of this study, in neonatal pigs orally inoculated with K88+ E coli, mortality was lower and the percentage of normal stool scores was higher in groups treated IM with ceftiofur sodium (3 or 5 mg ceftiofur per kg BW) compared to groups that received no ceftiofur treatment.
- Ceftiofur sodium (Naxcel/Excenel Sterile Powder; Pharmacia & Upjohn) administered IM at the dose labeled for the treatment of SRD appears to be an effective treatment for reducing losses due to neonatal pig diarrhea caused by K88+ enterotoxigenic strains of E coli.
Acknowledgments
The authors thank Trevor Preston and Rich Pounds for their technical support during the animal phase of this study.
References – refereed
1. Fairbrother JM. Neonatal Escherichia coli diarrhea. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:433-441.
2. Yancey RJ, Evans RA, Kratzer DD, Paulissen JB. Efficacy of ceftiofur hydrochloride for treatment of experimentally induced colibacillosis in neonatal swine. Am J Vet Res. 1990;53:349-353.
5. Food and Drug Administration Center for Veterinary Medicine. Good target animal study practices: clinical investigators and monitors. Rockville, Maryland: FDA; 1997. Guidance for industry #58.
6. Sarmiento JI, Casey TA, Moon HW. Postweaning diarrhea in swine: experimental model of enterotoxigenic Escherichia coli infection. Am J Vet Res. 1988;49:1154-1159.
7. SAS/STAT User’s Guide, Version 6. Vol 2. 4th ed. Cary, North Carolina: SAS Institute Inc; 1989.
8. Brown SA, Hanson BJ, Mignot A, Millérioux L, Hamlow PJ, Hubbard VL, Callahan JK, Kausche FM. Comparison of plasma pharmacokinetics and bioavailability of ceftiofur sodium and ceftiofur hydrochloride in pigs after a single intramuscular injection. J Vet Pharmacol Therap. 1999;22:35-40.
9. Salmon SA, Watts JL, Case CA, Hoffman LJ, Wegner HC, Yancey RJ. Comparison of MICs of ceftiofur and other antimicrobial agents against bacterial pathogens of swine from the United States, Canada, and Denmark. J Clin Microbiol. 1995;33:2435-2444.
10. Naxcel ceftiofur sodium sterile powder [package insert]. Kalamazoo, Michigan: Pharmacia & Upjohn; 2000.
11. Excenel RTU ceftiofur hydrochloride sterile suspension [package insert]. Kalamazoo, Michigan: Pharmacia & Upjohn; 2000.
14. Beconi-Barker MG, Hornish RE, Vidmar TJ, Dame KJ, Brown SA. Ceftiofur hydrochloride: plasma and tissue distribution in swine following intramuscular administration at various doses. J Vet Pharmacol Therap. 1996;19:192-199.
15. Gilbertson TJ, Jaglan PS, Koshy KT, Nappier JL, Stahl GL, Cazers AR, Nappier JM, Kubicek MF, Hoffman GA, Hamlow PJ. Environmental fate of ceftiofur sodium, a cephalosporin antibiotic. Role of animal excreta in its decomposition. Ag Food Chem. 1990;38:890-894.
16. Hornish RE, Kotarski SF. Cephalosporins in veterinary medicine – ceftiofur use in food animals. Curr Top Med Chem. 2002;2:717-731.
17. FDA/USDA/CDC. National antimicrobials resistance monitoring program: enteric bacteria. Veterinary Isolates 2000 Final Report. Joint Publication of the Food and Drug Administration Center for Veterinary Medicine, US Department of Agriculture and the Center for Disease Control; 2002.
References – non refereed
3. Hallberg JW, Hanson BJ. The efficacy of ceftiofur for the treatment of neonatal colibacillosis in swine. Proc 14th IPVS. Bologna, Italy. 1996;258.
4. Han JH, Roh IS, Jeong HK. Effect of Naxcel Sterile Powder in the treatment of colibacillosis in nursing pigs. Proc 14th IPVS. Bologna, Italy. 1996;274.
12. Fales WH, Ganjam IG, Wendell D, Karagiannis K, Turk JR, Miller MA, Pace LW, Kreeger JM, Turnquist SE, Johnson GC, Gosser HS. Minimal inhibitory concentration (MIC50-MIC90) antimicrobial susceptibilities of enteric and non-enteric pathogenic bacteria recovered from Missouri swine. Proc 41st Ann Meet Am Assoc Vet Lab Diagn. Minneapolis, Minnesota. October 1998.
13. Schiønning S, Millerioux L, Hewett GR. Determination of tissue concentrations of desfuroyl-ceftiofur in pigs after intramuscular injection of Excenel sterile powder [abstract]. Proc 6th EAVPT. 1994;6(2):76. Abstract 012.