Original research |
Peer reviewed |
Vaccine efficacy and immune response to Mycoplasma hyopneumoniae challenge in pigs vaccinated against porcine reproductive and respiratory syndrome virus and M hyopneumoniae
Tamara B. Boettcher, DVM, MS; Brad J. Thacker, DVM, PhD; Patrick G. Halbur, DVM, PhD; W. Ray Waters, DVM, PhD; Robert Nutsch, DVM, MS, MBA; Eileen L. Thacker, DVM, PhD
TBB, WRW: National Animal Disease Center, Ames, Iowa; BJT, PGH: Veterinary Diagnostic and Production Animal Medicine, Iowa State University, Ames, Iowa; RN: Pfizer Animal Health, Groton, Connecticut; ELT: Veterinary Microbiology and Preventive Medicine, Iowa State University, Ames, Iowa; Corresponding author: Eileen L. Thacker, VMRI, 2118 Veterinary Medicine Building, Department of VMPM, Iowa State University, Ames, IA 50011; Tel: 515-294-5097; Fax: 515-294-8500; E-mail: ethacker@iastate.edu.
Boettcher TB, Thacker BJ, Halbur PG, et al.Vaccine efficacy and immune response to Mycoplasma hyopneumoniae challenge in pigs vaccinated against porcine reproductive and respiratory syndrome virus and M hyopneumoniae. J Swine Health Prod. 2002;10(6):259-264. Also available as a PDF.
Summary
Objective: To investigate the efficacy and level of immune response induced by a Mycoplasma hyopneumoniae (MH) bacterin in pigs previously vaccinated with a modified live virus (MLV) porcine reproductive and respiratory syndrome (PRRS) vaccine and subsequently challenged with MH.
Methods: Pigs were vaccinated once with MLV PRRS vaccine (Day 0), twice with MH vaccine (Days 8 and 22), or both, and challenged with MH on Day 36. Necropsies were performed on Days 35 and 61-63. Efficacy of MH vaccine was determined by reduction in the percentage of lung affected by mycoplasmal pneumonia. Serum antibodies to PRRS virus (PRRSV) and MH, antibodies to MH in bronchoalveolar lavage fluid (BAL), and production of MH-specific interferon-gamma- (IFN-[gamma]-) secreting cells in tissues and peripheral blood were measured on Days 0 and 14 and at necropsy. Immunological assays included ELISAs for PRRSV and MH antibodies and an enzyme-linked immunospot assay for MH-specific IFN-[gamma]-secreting cells.
Results: Mycoplasma vaccine efficacy was not decreased by prior administration of PRRS vaccine. Serum MH antibody levels in pigs receiving both vaccines and challenged were numerically but not signifi-cantly higher than those in pigs receiving only MH vaccine and challenged. Pigs receiving both vaccines had significantly lower PRRSV sample:positive (S:P) ratios, but remained ELISA-positive (S:P>0.4). Levels of MH-specific IgG and IgA in BAL were significantly higher in both groups vaccinated for MH and challenged.
Implications: Administration of an MLV PRRS vaccine to MH-free pigs prior to vaccination with MH vaccine did not interfere with vaccine efficacy or immune responses to MH infection.
Keywords: Swine, Mycoplasma hyopneumoniae, porcine reproductive and respiratory syndrome virus, vaccine, antibodies
Search the AASV web site for pages with similar keywords.
Received: August 28, 2001
Accepted: February 22, 2002
Mycoplasma hyopneumoniae (MH), the cause of enzootic pneumonia, can be isolated from the majority of swine herds in the United States and is frequently isolated from pigs exhibiting clinical disease associated with porcine respiratory disease. Although pigs exhibiting respiratory disease are typically infected with multiple pathogens, diagnostic laboratories have found that MH, porcine reproductive and respiratory syndrome virus (PRRSV), and swine influenza virus (SIV) are among the most frequently detected pathogens in pigs (P. G. Halbur, written communication, 1996). The interaction between these pathogens is complex and plays a major role in the induction of pneumonia in pigs in the field. Previous studies have determined that while PRRSV infection did not increase the severity of mycoplasmal pneumonia, infection with MH potentiated the pneumonia induced by PRRSV, on the basis of clinical, macroscopic, and microscopic findings.1,2
Vaccination against MH is an important intervention strategy used to control mycoplasmal pneumonia in pigs. Although numerous studies have demonstrated that MH bacterins are protective against experimental challenge, vaccination failure in production settings is a frequent complaint.3-5 While there are a number of possible explanations for the failure of mycoplasma vaccines in the field, a recent study found that infection or administration of a modified live virus (MLV) PRRS vaccine at the time of MH vaccination appeared to significantly reduce the efficacy of the MH vaccine, as measured by the lack of significant reduction in the percentage of lung affected by pneumonia consistent with MH infection.6 It was also determined that while the efficacy of the MH bacterin was diminished, pigs experimentally infected with MH after inoculation with both a PRRS MLV vaccine and an MH bacterin had significantly increased levels of MH–specific antibodies in the serum and bronchoalveolar lavage fluid (BAL) compared to pigs that did not receive PRRS vaccine. The results of this study support previous reports that PRRSV infection is not systemically immunosuppressive, and may actually enhance antibody production to antigens.7, 8
Research has demonstrated that MH bacterins induce both a systemic antibody response, which is not correlated to protection, and a local antibody response consisting of both IgG and IgA.4,9 The correlation of local MH-specific antibody production and protection against pneumonia remains unclear, as a study by Djordjevic et al4 found no correlation between local antibodies and protection, while research in our laboratory demonstrated a positive relationship.9 However, increased levels of MH-specific local antibodies observed after MLV PRRS vaccination did not result in protection against mycoplasmal pneu-monia.6
The role of cellular immunity in protection against MH-induced pneumonia is also unclear. Lymphocyte responsiveness to mycoplasma antigens has been demonstrated by measuring the in vitro stimulation of lymphocytes after MH vaccination and experimental challenge.5,10 In addition, a recent study demonstrated that vaccination with a mycoplasma bacterin induced the production of MH-specific interferon-gamma (IFN-[gamma])-producing cells.9 Interferon-g is an important cytokine produced by helper cells, subtype 1 (TH1) lymphocytes and natural killer cells and is important for macrophage activation as well as protection against and clearance of viruses. These results suggest that a cellular immune response may play a role in protection against MH through the activation of macrophages.
The objective of this study was to assess the effect of prior administration of an MLV PRRS vaccine on the immune response induced by an MH bacterin and its ability to reduce the percentage of lung affected by mycoplasmal pneumonia. Antibodies specific for MH in serum and BAL and the number of IFN-[gamma]-producing cells in the peripheral blood, lung, and tracheobronchial lymph node were measured to assess the immune response to MH vaccination and infection. The percentage of lung affected by mycoplasmal pneumonia, determined by image analysis, was used to assess vaccine efficacy.
Materials and methods
Experimental animals, housing, and study design
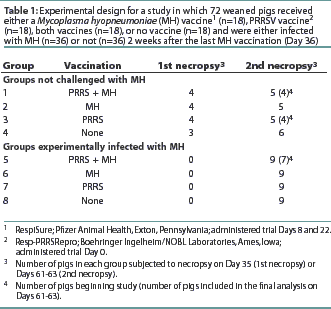
Seventy-two 2-week-old crossbred pigs procured from a herd serologically negative for PRRSV, SIV, and MH antibodies were randomly assigned to eight treatment groups with stratification by weight. The experimental design is summarized in Table 1. The study was conducted in accordance with the guidelines of the Iowa State University Institutional Committee on Animal Care and Use.
During the vaccination phase, the pigs were housed on raised decks in four rooms according to vaccination protocols. On trial Day 19, all pigs were moved to solid floors in the same rooms. All rooms used in the study had similar ventilation and lighting systems. All pigs were provided water and feed ad libitum throughout the trial.
Method and site of administration of vaccines
A commercial MLV PRRS vaccine (Resp-PRRSRepro; Boehringer Ingelheim/NOBL Laboratories, Ames, Iowa) was administered, according to the manufacturer’s recommendations, on trial Day 0 to Groups 1, 3, 5, and 7 (n=36), when the pigs were approximately 3 weeks of age. A commercial MH bacterin (RespiSure; Pfizer Animal Health, Exton, Pennsylvania) was administered according to the manufacturer’s recommendations on Days 8 and 22 to groups 1, 2, 5, and 6 (n=36). Rectal temperatures were measured on Day 0 and every other day for 10 observations in four pigs randomly selected from each group (1 to 8). Evidence of respiratory disease associated with PRRS, such as increased respiration rate or labored breathing, was assessed and scored immediately on a scale of 1 to 6 using a previously described system.11
Blood samples were obtained prior to each vaccination, prior to challenge, and at necropsy to measure antibody levels and to isolate peripheral blood mononuclear cells for use in the lymphocyte assays. On Day 35, four pigs were necropsied from Groups 1, 2, and 3, and three pigs from Group 4 (the unvaccinated control group). On Day 36, all pigs in Groups 5, 6, 7, and 8 were inoculated intratracheally with 10 mL of MH challenge inoculum, strain 232 (a derivative of strain 11), containing 105 color changing units per mL, as previously described.5All remaining pigs were necropsied on Days 61 and 63.
At necropsy, pigs were anesthetized with sodium pentobarbital administered intravenously and exsanguinated. Tissue samples were collected aseptically from the lungs, right inguinal lymph node, and right bronchial lymph node. Bronchial swabs were collected and cultured for swine respiratory pathogens, including Bordetella bronchisep-tica, Pasteurella multocida, Actinobacillus spp, Haemophilus spp, and Mycoplasma hyorhinis. Samples were inoculated onto blood agar and streaked with a Staphylococcus epidermidis nurse colony for support of A pleuropneumoniae and Haemophilus spp. Bacteria isolated were identified according to standard methods. Bronchial secretions were inoculated into Friis medium for isolation of MH. Lesions consistent with mycoplasmal pneumonia were sketched using standard lung diagrams. The proportion of lung area with lesions consistent with mycoplasma pneumonia was determined using a Zeiss SEM-IPS computerized image analyzing system which determines the total surface area of the lung affected by pneumonia.3 Macroscopic lesions consistent with PRRSV-induced pneumonia were determined using a method previously developed by Halbur et al.12 Tissue was frozen immediately for fluorescent antibody assay examination for mycoplasmal antigen.13 The lungs were lavaged with 50 mL of sterile phosphate buffered saline (PBS) for local antibody measurement using a previously described technique.9
ELISPOT assay for IFN-[gamma]
Mononuclear cells from peripheral blood, lung tissue, tracheobronchial and right inguinal lymph nodes, and tissues were isolated as previously described.5,14 Briefly, tissues were minced and passed through a 150-mesh stainless steel sieve to remove tissue fragments. Both tissue and peripheral blood mononuclear cells were diluted in PBS and isolated by differential centrifugation over Histopaque 1077 (Sigma Chemical, St Louis, Missouri). Cells were washed and resuspended in RPMI medium containing 10% fetal bovine serum, penicillin G (100 units per mL), streptomycin (100 mg per mL), and gentamicin sulfate (0.05 mg per mL), at a concentration of 2 x 106 cells per mL. The diluted cells were incubated with peroxidase-conjugated donkey anti-rabbit IgG antibody (Kirkegaard and Perry Laboratory, Gaithersburg, Maryland) and 3,3¢,5,5¢-tetramethylbenzidine membrane peroxidase (Sigma Chemical) was added as substrate. Spots corresponding to IFN-[gamma]-secreting cells were counted using a dissecting microscope, and data were reported as number of spot-forming cells per 2 x 105 lymphocytes. Lymphocytes were also isolated from the right inguinal lymph node and used as non-draining control samples for comparison to potential reactions in the tracheobronchial lymphocytes. Lymphocytes were cultured overnight with MH antigen, with concanavalin-A as a positive control, or with medium alone as a negative control.9,15
Serology and BAL analysis
Sera collected on Days -7, 14, 35, 61, and 63 were assayed for antibodies to MH and PRRSV.
The Tween 20 ELISA was used to measure MH antibodies as previously described.16 Known positive and negative sera and blank wells were included as controls on each plate. Readings greater than 2 SDs above the mean optical density (OD) value of the negative control (ie, OD values >0.023) were considered positive.
Serum antibodies to PRRSV were measured using a commercial ELISA kit (HerdChek PRRS; IDEXX Laboratories, Westbrook, Maine). Samples were considered seropositive for PRRSV if the sample: positive (S:P) ratio was >=0.4.
An ELISA assay was used to measure MH-specific antibodies in BAL as previously described.9 Plates were coated with a membrane preparation of MH clone 232-2A3.17 The specific antibody isotype was determined using peroxidase-labeled, heavy-chain-specific goat anti-swine IgA (Kirkegaard and Perry Laboratories), IgG, and IgM (Bethyl Laboratories, Montgomery, Texas). Results were reported as ODs.
Statistics
Analysis of variance was performed using the general ANOVA procedure of Statistix (Analytical Software, Tallahassee, Florida) to ascertain differences between treatment groups. All eight treatment groups were included in each analysis. If the P value generated by ANOVA was <.05, pair-wise comparisons among all eight treatment groups were performed by least significant difference at a significance level of P<=.05.
Results
Growth and health
No increased rectal temperatures or altered respiration consistent with PRRSV infection were observed in any pigs after vaccination with the MLV PRRS vaccine (data not shown). The average weight of all pigs was 7.1 +/- 0.9 kg on arrival, 29.6 +/- 2.9 kg on Day 35, and 51.1 +/- 4.5 kg at necropsy on Days 61-63. No differences in weight gain were found among the groups throughout the trial (P>.05). Four pigs were eliminated from the study due to chronic health problems, including lameness (2 pigs), rectal prolapse (1 pig) and Streptococcus suis-induced polyserositis (1 pig).
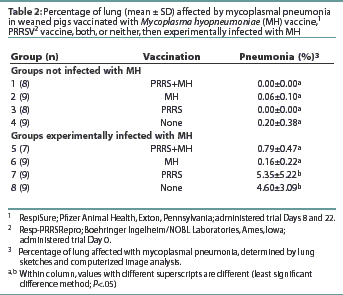
All pigs in Groups 7 and 8 had pneumonic lesions consistent with MH infection (Table 2). The average percentage of lung with mycoplasmal pneumonia was highest in Group 8, although no statistical difference (P>.05) in the average percentage of mycoplasmal pneumonia was observed between Groups 7 and 8. Groups 5 and 6 had lower averages (P<.05) of mycoplasmal pneumonia than observed in Groups 7 and 8. The average percentage of mycoplasmal pneumonia in Groups 5 and 6 did not differ (P>.05) from that in Groups 1, 2, 3, or 4 (non-challenged groups). No pneumonic lesions consistent with PRRS were observed in any pigs at either necropsy. All but one of the MH-challenged pigs were culture-positive for MH at necropsy (data not shown). Mycoplasma hyopneumoniae was not isolated from any of the non-challenged pigs. Several pigs in Groups 2 and 4 (non-challenged) had mild pneumonia inconsistent with mycoplasmal pneumonia. Culture of bronchial swabs revealed that these pigs were infected with Haemophilus parasuis. Immunofluorescence assay results detected MH antigen in the lung tissue of six of the 34 challenged pigs and none of the 34 unchallenged pigs (data not shown).
MH-specific IFN-[gamma]-producing cells
Minimal differences were observed between groups in the number of IFN-[gamma]-producing cells. No difference (P>.05) was observed between groups in the number of MH-specific IFN-[gamma]-secreting cells in any of the tissues at the first necropsy (data not shown). At the second necropsy, Group 5, with 7.7 +/- 13.0 spots per 105 cells, had more IFN-[gamma]-producing cells (P<.05) in the peripheral blood than Group 7, with 0.5 +/- 0.7 spots per 105 cells. Group 1, with 26.5 +/- 41.9 spots per 105 cells, had more IFN-[gamma]-secreting cells in the bronchiolar lymph nodes (P<.05) than any other group.
Serology and BAL analysis
All pigs were negative for MH antibodies upon arrival (data not shown). On Day 35, Groups 1, 2, 5, and 6 were seropositive for MH antibodies (Table 3). Although Groups 1 and 5 had numerically higher levels of MH antibodies than the other MH-vaccinated groups, no statistical difference was observed. On Days 61 and 63, the OD levels of MH antibodies had decreased in Groups 1 and 7, while the OD levels in Groups 5 and 6 had increased.
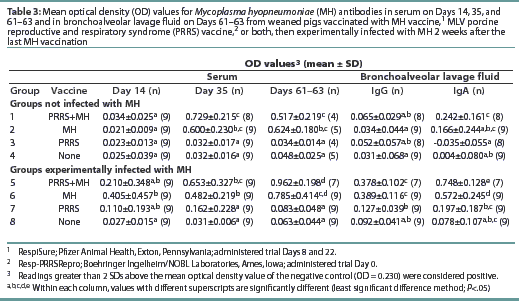
The MH OD levels in BAL prior to challenge at the first necropsy (data not shown) were low, and there were no differences between groups. At the final necropsy, both Groups 5 and 6 had increased mean OD levels of MH-specific IgG and IgA in the BAL as summarized in Table 3. Groups 5 and 6 had higher mean OD levels of MH-specific IgG and IgA than other groups. No other differences were observed among the groups. There were no differences in mean OD levels of MH-specific IgM antibodies among the groups at any time (data not shown).
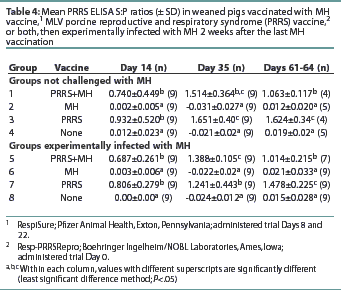
All pigs were seronegative for PRRSV antibodies upon arrival (data not shown). Groups 1, 3, 5, and 7 had S:P ratios >0.4 by Day 14, and the antibody levels remained >0.4 throughout the remainder of the trial (Table 4). Groups 2, 4, 6, and 8 remained PRRS-seronegative throughout the trial. Groups 1 and 5 had S:P ratios lower than Groups 3 and 7 at the final necropsy, although the S:P ratios of all PRRS-vaccinated groups remained >0.4.
Discussion
Pneumonia caused by MH remains a problem for the swine industry. Vaccination is a frequent and effective intervention strategy used to control the pneumonia induced by MH. However, practitioners in the field report frequent vaccine failures associated with commercial mycoplasmal vaccines. In a previous study, it was found that the presence of MH increased the duration and severity of PRRSV-induced pneumonia.2 A second study investigating the effect of vaccination on the potentiation of PRRSV-induced pneumonia by MH found that the presence of PRRSV, from either an MLV vaccine or infection, at the time of or within 2 weeks of mycoplasma vaccination, significantly reduced the ability of the mycoplasma vaccine to reduce the percentage of lung affected by mycoplasmal pneumonia.6 The results in that study suggest that timing mycoplasma vaccine in relation to PRRSV may be an important consideration in establishing successful vaccine programs. In order to further investigate the effect of PRRSV on the ability of an MH vaccine to reduce the percentage of lung affected by mycoplasmal pneumonia and the immune response to MH, pigs were vaccinated with an MLV PRRS vaccine prior to MH vaccination. In contrast to the earlier findings that the presence of PRRSV at the time of or after MH vaccination decreased vaccine efficacy, this study found that inoculation with an MLV PRRS vaccine prior to MH vaccination did not decrease the efficacy of the mycoplasma vaccine as determined by the percentage of lung affected by mycoplasmal pneumonia and serum antibody levels.
As found in the earlier studies, growth performance in pigs challenged experimentally with MH did not differ significantly between groups.5,9 The mycoplasmal pneumonia swine model used in our previous MH pathogenesis and vaccine studies, which utilized pigs with few other respiratory pathogens, housed in well-ventilated facilities and fed ad libitum, demonstrated no reduction in growth performance after experimental MH challenge. In addition, no clinical disease, as measured by rectal temperatures or altered respiratory rate, was observed after vaccination with the MLV PRRS vaccine in this study.
In addition to assessing the efficacy of the MH vaccine by macroscopic examination and determination of mean percentage of lung affected by mycoplasmal pneumonia, the potential impact of prior MLV PRRS vaccination on the immune response to the MH vaccine was assessed. A previous study in our laboratory had determined that MH vaccination without MH challenge induced the production of MH-specific IFN-[gamma]-producing cells.9 However, no increase in IFN-[gamma]-producing cells was observed after challenge with MH. Although less conclusive results were found in this study, the mean number of MH-specific IFN-[gamma]-producing cells was increased in the peripheral blood of pigs which received both MH and PRRS vaccines and were then challenged with MH. These results confirm the previous findings that MH vaccination induces the production of IFN-[gamma]-producing cells which may result in activation of macro-phages and thus aid in controlling the organism.
Previous studies have demonstrated that the presence of PRRSV may increase the production of antibodies to antigen.6-8 In an earlier study, the administration of an MLV PRRS vaccine between primary and secondary MH vaccinations increased MH antibody levels after the secondary mycoplasma vaccination, while in the study reported here, inoculation with an MLV PRRS vaccine prior to mycoplasma vaccination resulted in no significant differences in MH antibody levels. These results suggest that the impact of PRRSV on the immune system is complex and that the relative timing of PRRSV exposure and mycoplasma vaccination may be important for optimizing MH vaccine strategies.
All PRRSV-vaccinated pigs were seropositive for PRRSV antibodies by Day 14, and no difference between vaccine groups was observed. On Days 61-63, the PRRSV-vaccinated groups receiving MH vaccine had lower PRRS ELISA S:P ratios than the groups receiving only PRRSV vaccine. However, since the S:P ratios of all PRRSV-vaccinated groups remained >0.4, the impact of the MH bacterin on MLV PRRS vaccine efficacy is probably inconsequential.
To establish baseline data, several pigs from each group served as controls and were necropsied prior to challenge. Local antibody production, as measured in BAL, may be an important protective mechanism against mycoplasmal pneumonia, due to antibody location on the cilia of the respiratory tract. Minimal MH-specific IgG or IgA levels, as measured by OD, were present in the respiratory tract in MH-vaccinated pigs prior to challenge.9 However, this study confirmed that vaccination primes local lymphocytes of the respiratory tract to respond to challenge, as MH-specific IgG and IgA OD measurements in BAL were significantly greater in vaccinated groups compared to non-vaccinated groups. The increased levels of MH-specific IgG OD antibody levels in BAL of pigs inoculated with both PRRS and MH vaccines and then challenged with MH were also consistent with our earlier study.6 Whether the increased MH-specific IgG levels present in the BAL in PRRS-vaccinated pigs reflect increased IgG levels in the serum or from lymphocytes localized to the lung parenchyma is currently unknown. However, it is thought that IgA antibodies are important in control of pathogens on the mucosal surface, while IgG is more active in the lung parenchyma.
Mononuclear cells producing IFN-[gamma] were measured to assess the effect of MLV PRRS vaccination on the cellular immune response induced by MH vaccination. The presence of IFN-[gamma] enhances the activity of NK cells and macrophages as well as increasing major histocompatibility complex expression on the cell surface, which is important in antigen presentation and aiding B- and T-cell interactions. Interestingly, in pigs vaccinated with MLV PRRS vaccine and then challenged with MH, there was an increased mean number of cells secreting IFN-[gamma] in the peripheral blood. These results parallel the increase in MH antibody levels observed in conjunction with PRRSV infection and present further evidence of the complexity of PRRSV impact on the immune system. Lymphocytes from lung tissue and the inguinal lymph node did not produce increased numbers of IFN-[gamma]-secreting cells in response to any vaccine strategy or challenge. There were some inconsistencies in the ELISPOT assay that may have affected the results. The results of this study and those of our previous study indicate that the timing of PRRSV vaccination, infection, or both is important for MH vaccine efficacy.6 The mechanism by which PRRSV decreased the ability of the MH vaccine to reduce mycoplasmal pneumonia is unknown and confirms that the interaction and effect of PRRSV and MH on the respiratory tract is complex, and many questions remain. However, on the basis of the results of these two studies, timing of vaccination in relation to PRRSV infection may be important to ensure vaccine efficacy. The results of these studies should provide additional information to swine veterinarians and producers to assist in establishing effective vaccination programs for the control of swine respiratory disease.
Implications
- Vaccination with an MLV PRRS vaccine prior to administration of an MH vaccine did not decrease the ability of a commercial MH vaccine to reduce experimental mycoplasmal pneumonia.
- Average MH antibody levels were higher in pigs challenged with MH after vaccination first with an MLV PRRS vaccine and then with MH vaccine than in pigs challenged with MH after vaccination with MH vaccine alone.
- The average number of MH-specific INF-[gamma]-producing lymphocytes was greater in the peripheral blood of pigs vaccinated with both PRRS and MH vaccines than in pigs vaccinated with MH vaccine alone.
- The average MH-specific IgG levels in BAL fluid were higher in pigs vaccinated with both PRRS and MH vaccines than in pigs inoculated with MH vaccine alone.
Acknowledgements
The authors wish to acknowledge Nancy Upchurch, Barbara Erickson, Theresa Young, Kris Ruebling, and Michelle Penland for technical assistance, and William Christopher for statistical consulting. Interferon-gamma reagents were supplied by Dr Frederico Zuckermann, University of Illinois, Urbana-Champaign. Supported by a grant from the National Pork Producers Council.
References – refereed
1. Van Alstine WG, Stevenson GW, Kanitz CL. Porcine reproductive and respiratory syndrome virus does not exacerbate Mycoplasma hyopneumoniae infection in young pigs. Vet Micro. 1996;49:297-303.
2. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Micro. 1999;37:620-627.
3. Ross RF, Zimmermann-Erickson BJ, Young TF. Characteristics of protective activity of Mycoplasma hyopneumoniae vaccine. Am J Vet Res. 1984;45:1899-1905.
4. Djordjevic SP, Eamens GJ, Romalis LF, Nicholls PJ, Taylor V, Chin J. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing denatured membrane antigen pool and adjuvant. Aust Vet J. 1997;75:504-511.
6. Thacker EL, Thacker BJ, Young TF, Halbur PG. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine. 2000;18:1244-1252.
7. Albina E, Piriou L, Hutet E, Cariolet R, L’Hospitalier R. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV). Vet Immunol Immunopath. 1998;61:49-66.
8. Bautista E, Molitor T. Cell mediated immune responses to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997;10:83-94.
9. Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Mucosal and systemic characteristics of protective activity of a Mycoplasma hyopneumoniae bacterin. Am J Vet Res. 2000;61:1384-1389.
10. Kristensen B, Paroz P, Nicolet J, Wanner M, de Weck AL. Cell-mediated and humoral immune responses in swine after vaccination and natural infection with Mycoplasma hyopneumoniae. Am J Vet Res. 1982;42:784-788.
11. Halbur PG, Paul PS, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparative pathogenicity of nine US porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a five-week-old cesarean-derived, colostrum-deprived pig model. J Vet Diagn Invest. 1996;45:11-20.
12. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Path.. 1995;32:648-660.
13. Amanfu W, Weng CN, Ross RF, Barnes HJ. Diagnosis of mycoplasmal pneumonia of swine: sequential study by direct immunofluorescence. Am J Vet Res. 1984;45:1349-1352.
14. Thacker EL, Fulton JE, Hunt HD. In vitro analysis of a primary, major histocompatibility complex (MHC)-restricted, cytotoxic T-lymphocyte response to avian leukosis virus (ALV), using target cells expressing MHC class I cDNA inserted into a recombinant ALV vector. J Virol. 1995;69:6439-6444.
15. Zuckermann FA, Husmann RJ, Schwartz R, Brandt J, Mateu de Antonio E, Martin S. Interleukin-12 enhances the virus-specific interferon gamma response of pigs to an inactivated pseudorabies virus vaccine. Vet Immunol Immunopath. 1998;63:57-67.
16. Bereiter M, Young TF, Hoo HS, Ross RF. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Micro. 1990;25:177-192.
17. Messier S, Ross RF. Interactions of Mycoplasma hyopneumoniae membranes with porcine lymphocytes. Am J Vet Res. 1991;52:1497-1502.