Original research |
Peer reviewed |
A case-control study investigating risk factors associated with postweaning Escherichia coli diarrhea in southern Ontario
Rocio Amezcua, DVM, EPA, MSc; Robert Friendship, DVM, MSc, Diplomate ABVP; Catherine Dewey, DVM, MSc, PhD; Carlton Gyles, DVM, MSc, PhD
RA, RF, CD: Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario. CG: Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, Ontario. Corresponding author: Dr Robert Friendship, Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario, N1G 2W1; Tel: 519-824-4120, ext 4022; E-mail: rfriends@uoguelph.ca.
Amezcua R, Friendship R, Dewey C, et al. A case-control study investigating risk factors associated with postweaning Escherichia coli diarrhea in southern Ontario. J Swine Health Prod. 2002;10(6):245-249. Also available as a PDF.
Summary
Objectives: To investigate management, housing, and nutrition factors and potential viral infections that might be associated with postweaning Escherichia coli diarrhea in pigs in southern Ontario.
Methods: A case-control study involving 50 herds was conducted. Information on hygiene, management, facilities, management of diarrhea, porcine reproductive and respiratory syndrome virus (PRRSV) status, and nursery feed management was collected through a questionnaire. Rectal swabs and sera collected from ten weaned pigs in each herd were tested for F4+ (K88+) E coli and antibodies to two viral pathogens. Hemolytic E coli-like colonies were tested for agglutination in specific anti-F4 antiserum.
Results: Twenty-six herds were designated as cases and 22 as controls. Escherichia coli positive for F4 were isolated from pigs in 15 case herds and 3 control herds. Postweaning diarrhea did not occur in the control herds during the study. In case herds, there were more feeder spaces per pen, the first rations offered in the nursery were more likely to be pelleted feed, and nursery pigs and gestating sows were more likely to be vaccinated against PRRSV than in control farms.
Implications: Weaning age, weaning weight, and commingling of pigs, which are commonly considered risk factors for postweaning diarrhea, did not appear to be contributing factors in this study. Vaccination for PRRSV was associated with an increased risk of postweaning diarrhea, which occurred in a variety of herd types and sizes.
Keywords: swine, Escherichia coli, postweaning diarrhea, nursery management, starter feed
Search the AASV web site for pages with similar keywords.
Received: August 31, 2001
Accepted: February 25, 2002
Swine practitioners noted an increase in the number of cases of postweaning diarrhea and mortality associated with F4+ (K88+) Escherichia coli occurring in Ontario beginning in the fall of 1997.1 The total number of swine enteric submissions to the Animal Health Laboratory (AHL; Guelph, Ontario) between October and May remained constant at approximately 23% of total swine submissions. However, in that period, the total number of F4+ E coli isolates from piglets increased from 20% of total enteric submissions in 1996-1997 to 42% in 1997-1998.1 Information accompanying diagnostic laboratory submissions indicated that the infection sometimes progressed so rapidly that pigs of 2 to 8 weeks of age were found dead before clinical signs were observed. In epidemics of postweaning diarrhea, up to 70% of all pigs may show clinical signs,2 and the group mortality rate may reach 25% in the absence of adequate medication.3
There is no doubt that toxigenic E coli is involved in the pathogenic process of postweaning digestive disorders,4 but may also be found in healthy piglets raised on farms with no history of postweaning diarrhea.5 The presence of an infectious agent is not sufficient to induce the clinical manifestations of enzootic health disorders.6 Full expression of diseases such as postweaning colibacillosis not only requires sufficient infection pressure, but also is strongly dependent on environmental and management conditions. It is evident that additional factors are required for the onset of clinical signs of postweaning diarrhea.5-8 It has been suggested that postweaning F4+ E coli diarrhea frequently appears in commingled, multi-source early-weaned pigs.1 Dietary change, which necessarily occurs with segregated-early-weaning management, may play an important role in path-ogenesis of the disease; in addition, there may be a build-up of enterotoxigenic E coli (ETEC) organisms in the environment.6-8 Infection with rotavirus or transmissible gastroenteritis (TGE) virus may predispose pigs to postweaning diarrhea.9 In herds where clinical porcine respiratory and reproductive syndrome (PRRS) is prevalent, E coli is often involved in secondary infections.10 We investigated management, housing, nutrition, and viral infections (PRRS and TGE) as potential factors associated with the resurgence of postweaning E coli diarrhea (PWECD) in Ontario.
Materials and Methods
Selection of the farms
A total of 50 farms were visited in the summer of 1999 as part of a case-control study. Case and control herds were selected from the records of the AHL, with the assistance of several swine practitioners. Criteria for selection of herds as cases or controls included history of F4+ E coli infection in the herd (present or absent) and a history of diarrhea or diarrhea and sudden death in the previous year (March-December 1998). Twenty-five herds in each category were sought in March of 1999. Herds were classified as case herds if clinical signs of E coli diarrhea and mortality occurred in postweaning pigs, and F4+ E coli was isolated. Herds were classified as control herds if there was no history of PWECD and the disease was not diagnosed at the time of the visit. These herds were randomly selected from those submitting samples to the AHL during the same period as the case herds, but where a diagnosis of F4+ Ecoli was not made.
Survey information
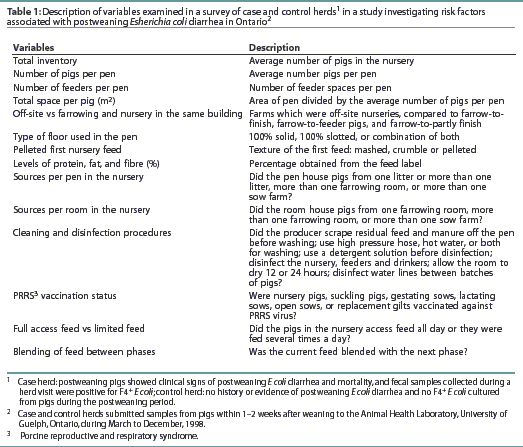
Information on farm hygiene, nursery management, nursery facilities, management of postweaning diarrhea, PRRS virus (PRRSV) status, and feed management in the nursery was collected through a survey. Definitions of variables included in the survey are listed in Table 1. Factors in addition to those described in Table 1 included producer-reported average daily gain in the nursery, average weaning weight, average age at weaning, continuous flow management, and all in-all out by room.
Sample collection
Rectal swabs were collected from ten weaned pigs in each herd at the time of the herd visit to ensure consistency in culturing for the presence of F4+ E coli. Sample size was calculated on the basis of a herd prevalence of 36% using a 99% confidence interval for a population of 1000 animals. Samples were collected 1 to 2 weeks after weaning from pigs that showed clinical signs of diarrhea. In herds where no diarrhea occurred, pigs were sampled in a random manner from the same age group. Depending on the size of the herd, one or two samples were taken from each pen. Samples were submitted to Gallant Custom Laboratories Inc, Cambridge, Ontario, for isolation of E coli. Slide agglutination tests for F4 antigen and for O and K serogroups implicated in PWECD were performed on hemolytic E coli isolates.
Serum samples were obtained from the same ten weaned pigs in each herd, using the orbital sinus method for blood collection.11 Blood samples were refrigerated overnight, then centrifuged the following day. Serum was separated and stored at -70°C. Sera were tested at the AHL for antibodies to TGE and PRRSV. Results were used to determine whether there was an association between these diseases and PWECD status. A virus neutralization test was used as a screening test on all sera to detect coronavirus antibodies, and then a blocking ELISA (Svanovir; Svanova Biotech Uppsala, Sweden) was used to differentiate TGE from respiratory corona- virus antibodies. The IDEXX HerdChek ELISA (IDEXX Laboratories Canada Corporation, Toronto, Ontario) was used to detect PRRSV antibodies. Herds were grouped by PRRS serological status as follows: serologically negative: sample:positive (S:P) ratio <0.4 in all samples; low S:P ratios: S:P ratio 0.4 to 2.5 in at least one sample; high S:P ratios: S:P ratio >2.5 in at least one sample. Low S:P ratios (as defined) are usually a result of maternal antibodies, past infection, or vaccination. High S:P ratios (as defined) imply recent field infection, usually referred to as active PRRSV infection.
Statistical analysis
The simple associations between case and control herd status and management and disease factors were determined using a chi-square test for qualitative variables, and a Fisher’s exact test or a Student t test for quantitative variables. Fisher’s exact test was used in cases when expected values in the two-by-two table were <5 in at least one of the cells. Odds ratios (OR) of qualitative variables and their confidence intervals were calculated. A value of P<.05 was considered significant; P values between .06 and .1 were considered numerically reportable as potential trends. Risk factors related to the case and control classification (P<.20) were re-examined in a multivariate model using logistic regression. Models were built using backwards elimination: those with the highest P values were removed one at a time until all factors left in the model were statistically significant at P<.05. Statistical analysis was completed in Statistix, (Statistix7, Version 1.0, 1996; Analytic Software, Tallahassee, Florida) and SAS System Program (Version 8.2; SAS Institute Inc, Cary, North Carolina).
Results
In three herds originally selected as controls, pigs developed postweaning diarrhea and were diagnosed with F4+ E coli just before the farm was visited. These three herds were designated as cases. One case herd was dropped from the study because the survey was not completed at the time of the visit, and a second case herd was dropped because it was a grower-finisher operation. Finally, 26 herds were designated as case herds and 22 as control herds. Escherichia coli positive for F4 was cultured from pigs in 15 of the 26 case herds, and in three of the 22 control herds, but PWECD did not occur in the control herds. Case herds were 8.6 times more likely to yield positive culture results for F4+ E coli than control herds (P=.002).
Nursery inventory ranged from 20 to 4200 pigs (1099.2 +/- 1086) in case herds and from 60 to 3000 pigs (1294 +/- 926.2) in control herds. There was no difference in size between case and control herds (P>.05). Management systems used in both case and control herds included farrow-to-finish, farrow-to-partly finish (where only a portion of grower-finisher pigs were raised to market on the farrowing site), farrow-to-feeder, and off-site nurseries.
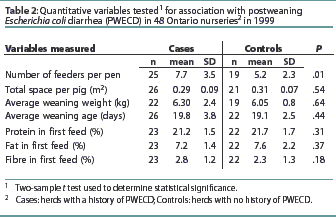
There were no differences between case and control herds in the following parameters: population density, average age at weaning, weight at weaning, and levels of protein, fat, and fiber in the first feed offered to the pigs in the nursery (Table 2). No differences (P>.05) were found between cases and controls for the following variables: multiple sources of pigs per pen or room; type of flooring; disinfection procedures; use of creep feed; or use of all in-all out compared to continuous flow management by room or building.
Case herds commonly reported changes in feeding and management in response to diarrhea. The common feed changes reported were purchasing feed from a different supplier; changing the in-feed medication; offering a limited amount of feed several times per day; blending of feed between phases; decreasing the level of protein; and increasing the level of fiber. The common management changes reported were increasing weaning age; improving control of temperature and ventilation; creation of sick pens; decreasing density in pens; and reducing the mixing of pigs.
Case herds had more feeder spaces per pen than control farms (Table 2). Case herds were more likely to use pelleted and crumbled feed in the first ration offered in the nursery, compared to control herds (Table 3). Control herds more commonly used detergent prior to disinfection than case herds (Table 3). The type of feed in the first nursery ration (mash or pelleted feed) and the use of detergent before disinfecting were tested for interaction within PRRS-positive and PRRS-negative herd status. No interaction was found between PRRS status and either the use of detergent (P>.05) or the type of feed in the first nursery ration (P>.05).
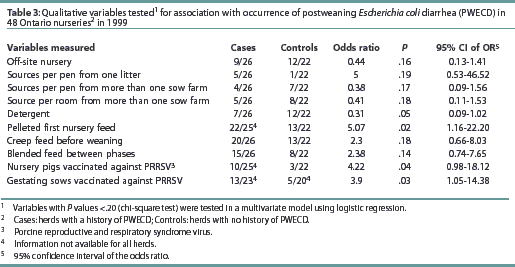
Among the 26 case herds, seven were serologically negative for PRRSV; 17 herds had low S:P ratios (between 0.4 and 2.5), possibly resulting from vaccination or passive immunity; and two herds had high S:P ratios (>2.5), indicative of recent PRRSV infection. Among the 22 control herds, six were serologically negative for PRRSV, 16 had low S:P ratios, and none had high S:P ratios indicative of active PRRSV infection. There was no association between PRRS serological status and occurrence of PWECD (P=.4). However, case herds were more likely to vaccinate their nursery pigs against PRRSV than control herds (Table 3). The only PRRS vaccine used in both case and control herds was Ingelvac PRRS MLV (Boehringer Ingelheim, Burlington, Ontario). Case herds were more likely than control herds to vaccinate gestating sows against PRRSV (Table 3). In PRRS-positive herds, after controlling for sow PRRS vaccination, nursery vaccination was not important as a risk factor for E coli. After controlling for nursery vaccination, sow vaccination was also not important as a risk factor for E coli. These factors were confounded, ie, associated with one another (P=.008).
Two of the 26 case herds and one of the 22 control herds were serologically positive for TGE (coronavirus differential ELISA test). No association was found between PWECD and positive TGE titres (P=.6).
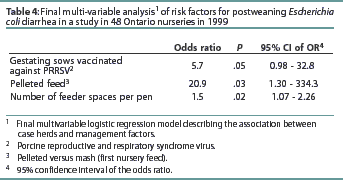
In the final-multivariable model, case herds were more likely than control herds to use PRRS vaccine in sows, case herds were more likely than control herds to feed pelleted feed in the nursery rather than mash, and case herds allowed more feeder spaces per pen than control herds (Table 4).
Discussion
Culture of rectal swabs from scouring piglets is an effective method of detecting F4+ E coli when clinical disease is present. However, some of the case herds were visited at a time when diarrhea was not a clinical problem. Isolation of F4+ E coli from nursery pigs in only 15 of the 26 case herds (57%) at the time of the herd visit reflects the sporadic occurrence of the disease.
The wide range in the estimates of the confidence intervals of the odds ratios for qualitative variables in this study might be due to the small sample size of herds included. There were no differences between case and control herds for a number of factors commonly thought to be associated with nursery pig diseases.6-8 In particular, early weaning age and commingling of pigs from several herds into one nursery room, practices commonly associated with segregated-early-weaning production systems, were not associated with a higher likelihood of PWECD. In this study, multi-site and single-site herds were represented in case and control herds in approximately equal proportions, and weaning weights and ages were similar between case and control herds. Weaning at an early age has been considered a risk factor for PWECD because the physiological immaturity of the gut allows undigested substrate to pass along the digestive tract and supply nutrients to pathogenic bacteria.12 In addition, immunity is not fully developed in the very young pig, which relies on passive protection from colostrum and milk.
Differences between case and control herds included feeding pellets or mash as the first nursery feed, and number of spaces at the feeders. It has been suggested that heat-treated cereals, for example, in pelleted feed, might provide a better environment for E coli proliferation.13 It is believed that pelleted feed provides more nutrients for E coli in the following two ways: first, pigs eat more of the pelleted feed; and second, pelleted feed moves through the gut more rapidly so that fewer of the nutrients are absorbed by the pig, leaving more for the organisms. The results of this study support this concern that pelleted feed is a risk factor for PWECD. Greater feeder space availability might allow or encourage pigs to overeat after weaning, which may contribute to diarrhea.14 Feed restriction has been advocated as a method of reducing PWECD.3,9,14 However, other workers have suggested that reduced feeder space is a risk factor for PWECD.6
Our study supports the view that unlimited access to feed in the early weaning stage is a risk factor for PWECD. Hygiene was expected to be a major risk factor for PWECD, with poor cleaning procedures resulting in increased bacterial challenge. Most farms cleaned pens between groups of pigs whether or not E coli was a problem. Power washing with a surfactant reduces bacteria and enables emulsification of the organic matter that protects the pathogens.15 More control farms used a detergent prior to disinfection, and therefore control herd facilities might have been cleaner compared to case herd facilities, but on the basis of visual inspection, the nurseries in both groups appeared similar in cleanliness. In all likelihood, pigs carry F4+ E coli into the nursery in intestinal flora,16 and therefore PWECD may occur no matter how thorough the cleaning procedure is.
Use of PRRS vaccine has been associated with the birth of weak viremic pigs,17 and clinical PRRS may exacerbate E coli problems.10,18 There is some biological basis to support the suggestion that vaccination of pregnant sows or weanling pigs might reduce gut immunity and contribute to E coli diarrhea, and this association should be further investigated.
Many factors associated with PWECD in experimental studies were not significant in this observational herd level study. This is consistent with the view that multiple risk factors must be present, as well as F4+ Ecoli, in order for clinical diarrhea to occur. In herds in which PWECD did not occur despite younger weaning age or other possible risk factors, undocumented procedures may have been counteracting the negative influences. It is also possible that in at least some of the control herds, risk factors for PWECD were present, but F4+ E coli was not.
Implications
- Weaning age, weaning weight, and commingling of pigs from multiple sources were not risk factors for PWECD in this case-control study.
- Vaccination for PRRS appeared to be associated with an increased risk of PWECD.
- Pelleted feed and larger number of feeder spaces were associated with an increased risk of PWECD.
- PWECD occurred on a variety of farm types and sizes.
References – refereed
3. Bertschinger HU. Postweaning Escherichia coli diarrhea and edema disease. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press. 1999:441-454.
4. Van Beers-Schreurs HM, Vellenga L, Wensing T, Breukink HJ. The pathogenesis of the postweaning syndrome in weaned piglets: a review. Vet Q. 1992;14:29-34.
9. MacKinnon JD: Enteritis in the young pig caused by Escherichia coli. Pig J. 1998;41:227-255.
10. Done SH, Patron DJ. Porcine reproductive and respiratory syndrome: clinical disease, pathology and immunology suppression. Vet Record. 1995;136:32-35.
11. Huhn RG, Osweiller GD, Switzer WP. Application of the orbital sinus bleeding technique to swine. Lab Animal Care.1969;19:403-405.
13. Lawrence TLJ. Processing and preparation of cereals for pigs diets. In: Cole DJA, Haresign W, Garnsworthy PC, eds. Recent Developments in Pig Nutrition. Loughborough, Leicestershire: Nottingham University Press. 1985:230-245.
14. Hampson DJ, Smith, WC. Influence of creep feeding and dietary intake after weaning on malabsorption and occurrence of diarrhea in the newly weaned pig. Res Vet Sci. 1986;41:63-69.
16. Hampson DJ, Fu AF, Robertson ID. Investigation of the source of hemolytic Escherichia coli infecting weaned pigs. Epidem Infect. 1987;99:149-153.
17. Mengeling WL, Lager KM, Vorwald AC. Safety and efficacy of vaccination of pregnant gilts against porcine reproductive and respiratory syndrome. Am J Vet Res. 1999;60:796-801.
18. Nakamine M, Kono Y, Abe S, Hosino C, Shirai J, Ezaki T. Dual infection with enterotoxigenic Escherichia coli and porcine reproductive and respiratory syndrome virus observed in weaning pigs that died suddenly. J Vet Med Sci. 1998;60:555-561.
References – non refereed
1. Josephson G, Smart N. K88 strains of E coli. Anim Health Lab Newsl. 1998;2:2.
2. Kniffen T, Neumann E. Field experience with E coli K88. Workshop #7. Emerging Nursery Pig Diseases and Management Solutions. Proc AASP Workshop #7. Indianapolis, Indiana. 2000:31-34.
5. Fairbrother JM. Identification, nomenclature, and diagnosis of pathogenic Escherichia coli. Ann Meet WCASP Proc. 1999:21-31.
6. Madec F, Buddle JR. A reflection on the strategies for tackling multifactorial disease problems in pigs with specific reference to postweaning enteric disorders. Proc IPVS Cong. Ames, Iowa. 2002:1:113-120.
7. Madec F, Leon E. The role of management and husbandry in pig health, with emphasis on post-weaning enteric disorders. Manipulating Pig Production VII Symposium. Austr Pig Science Ass Conf. 1999:200-209.
8. Löfstedt M, Holmgren N, Lundeheim N. Risk factors for post-weaning diarrhoea in Swedish pig herds. Proc IPVS Cong. Melbourne. 2000:367.
12. Josephson G, Smart N, McEwen B, Gough J. K88+ E coli diarrhea in post-weaning pigs. Animal Health Laboratory, University of Guelph. Ann Centralia Swine Res Update. 1999:38-39.