Diagnostic notes
Non refereed
Enterotoxigenic Escherichia coli infection in pigs and its diagnosis
David H. Francis, PhD
Department of Veterinary Science, South Dakota State University, Brookings, SD 57007-1396.
Francis DH. Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. J Swine Health Prod. 2002;10(4):171-175. Also available as a PDF.
Enterotoxigenic Escherichia coli (ETEC) is a major cause of illness and death in neonatal and recently weaned pigs. However, pigs older than approximately 8 weeks appear to be resistant to infection. Strains of ETEC that cause diarrhea in pigs possess two types of virulence factors, adhesins and enterotoxins, both of which are essential for disease to occur.
Fimbrial adhesins
Porcine ETEC isolates produce any of five different adhesins, all of which are fimbriae (also known as pili): K88 (F4), K99 (F5), 987P (F6), F41 (F7) and F18.1,2 Fimbrial adhesins K88 and F18 occur in several antigenic forms. The K88 fimbrial variants include K88ab, K88ac, and K88ad. By far the most prevalent, if not the only form of K88 found in the United States, is K88ac.3 Variants of F18 include F18ab and F18ac. Strains expressing F18ab may be more commonly associated with edema disease than strains expressing F18ac. Adhesins of the F41 type are most often found in association with K99, and their significance in naturally occurring disease is uncertain.
The fimbrial adhesins target specific receptors on porcine intestinal brush border epithelial cells (enterocytes), enabling the bacteria to colonize the cell surface and there excrete toxins whose action includes production of diarrhea in the host animal. Susceptibility of pigs to ETEC is in large part a function of expression and exposure of these receptors on the intestinal luminal surface. Clinically apparent infection of pigs by ETEC is restricted both by animal age and animal lineage.1,4,5 Lineage-restricted susceptibility of pigs to ETEC and, at least in some cases, age-restricted susceptibility, is associated with availability of receptors for the attachment of fimbrial adhesins.5
Enterotoxins
Toxins produced by ETEC strains that cause diarrhea in pigs include heat labile enterotoxin (LT), heat stable enterotoxin type A (STa); heat stable enterotoxin type B (STb); Shiga toxin type 2e (Stx2e), and enteroaggregative E coli heat-stable enterotoxin 1 (EAST1).1,4,6 Enterotoxin Stx2e, also known as edema disease factor, is the cause of lesions associated with edema disease in pigs. When absorbed into the blood, this toxin destroys endothelial cells in small vessels, resulting in blood clots, hemorrhage, ischemic necrosis, and edema in vital organs, including the brain.7,8 The significance of EAST1 in porcine diarrheal disease is not known.
Patterns of virulence determinants
Virulence determinants are not randomly distributed among virulent strains, but typically occur in patterns associated with specific serogroups and fimbriae. Clustering of virulence determinants around serogroups, and the ages of pigs commonly infected with ETEC of each virulence type, are shown in Table 1. Almost all strains of ETEC isolated from weaned pigs with diarrhea contain the gene for STb. Near universal presence of this gene in postweaning diarrhea isolates strongly suggests that STb plays an important role in pathogenesis that is not duplicated by other enterotoxins. However, very few ETEC strains exhibit only STb genes. This suggests that other enterotoxins are also important in postweaning diarrheal disease. In addition to STb genes, postweaning isolates also typically possess genes for STa, LT, or both.
Patterns of age-associated susceptibility to various ETEC strains
The vast majority of pigs clinically infected with ETEC strains expressing K99 or 987P are neonates, although pigs may be susceptible to these strains for a week or more after birth.1 Receptors for K99 fimbriae are displayed at diminished concentrations as pigs mature beyond the neonatal stage, suggesting that susceptibility to ETEC expressing these fimbrial adhesins is a function of receptor expression.9 Receptors for 987P appear to be expressed in abundance in adult animals, suggesting that a lack of receptors is not the reason for loss of susceptibility of pigs to 987P+ ETEC.10 Receptors for K88 and F18 also appear to be abundant in adult animals, again suggesting that cessation of receptor expression is not the cause of age-associated resistance of older pigs to ETEC expressing these fimbriae.11 It is unlikely that age-associated resistance to 987P+, K88+ or F18+ ETEC is in consequence to immunity developed from natural exposure to the organisms, as exposure to 987P+ ETEC and K88+ ETEC would likely occur at the same age, yet the age at which resistance is developed is distinctly different for each strain. There is some evidence in mice for development of resistance to STa enterotoxin with age.12 Perhaps pigs also develop age resistance. However, there seems to be little correlation between the type of enterotoxin produced by an ETEC strain and the age at which pigs become resistant. In addition, adult humans are susceptible to traveler’s diarrhea caused by ETEC. This indicates that at least some adult animals are not resistant to all types of E coli enterotoxins. It is possible that changes in the intestinal brush border glycocalyx associated with animal aging render fimbrial receptors less available for bacterial attachment. As receptors for the different fimbrial adhesins are different in size and are probably different from each other in their presentation on the enterocyte surface, age-associated or diet-associated changes in the glycocalyx might mask receptors of different fimbriae at different animal ages.11,13
Receptors for F18 fimbriae are not expressed in neonatal animals and may not appear in sufficient numbers to allow F18+ ETEC infection until about the time when most pigs are weaned.14 Lack of susceptibility of neonatal pigs to F18+ ETEC strains probably is in consequence to a lack of F18 receptors.
Patterns of lineage-associated susceptibility to various ETEC strains
In addition to age-associated resistance of pigs to K88+ and F18+ ETEC strains, lineage-associated resistance to infection by these strains also exists. In each case, susceptibility is an autosomal dominant trait, and resistance is in consequence of failure of the animal to express the required receptors on enterocytes. When purebred pigs of four breeds (Chester White, Duroc, Hampshire, and Yorkshire) were tested for expression of K88 receptors, 46 of 96 animals (48%) expressed the receptors necessary for susceptibility to K88ac, the major K88 variant found in the United States.15 There was substantial variation in the prevalence of susceptible animals among the breeds, but the significance of this variation could not be determined due to the small sample size. The prevalence of pigs of the F18 susceptibility phenotype has not been determined, but casual observation would suggest that it is considerably higher than that of K88 ETEC susceptibility.
K88 and F18 receptors on porcine enterocytes
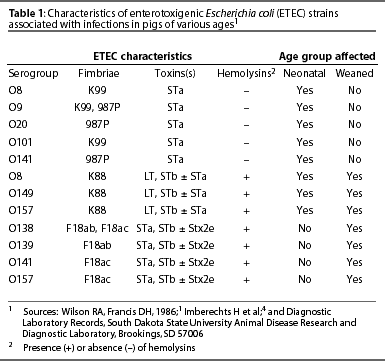
The receptor for K88ab and K88ac in porcine enterocytes has been identified as a mucin-type sialoglycoprotein (IMTGP). This mucus-like molecule is anchored to the brush border surface of enterocytes.11 Only pigs that express IMTGP are susceptible to K88ab+ and K88ac+ ETEC, and susceptibility of young pigs to K88+ ETEC is highly correlated with expression of IMTGP.5 The presence of IMTGP in porcine enterocytes is also correlated with attachment of K88ab+ and K88ac+ bacteria to brush border vesicles prepared from pig enterocytes collected by biopsy or at euthanasia. Thus, the brush border adherence assay is a useful test for K88+ ETEC susceptibility-resistance phenotypes of pigs (Figure 1). A genetic marker of K88+ ETEC resistance-susceptibility has not been identified, although the resistance-susceptibility determinant appears to be on chromosome 13.16
The receptor for F18ab and F18ac has not been identified, but a genetic marker for loss of receptor expression was shown closely linked to the marker for porcine stress syndrome and porcine blood group inhibitor (S). The marker was mapped to an alpha (1,2) fucosyltransferase (FUT) gene on chromosome 6; namely, that for FUT1.17 Differences between susceptible and resistant FUT1 gene open reading frames (ORFs) were polymorphisms at base pairs 307 and 857, which resulted in amino acid substitutions at positions 103 (threonine substituted for alanine) and 286 (glutamine substituted for argenine). Animals resistant to F18 ETEC are homozygous for threonine at amino acid 103 of the FUT1 enzyme. Levels of FUT enzyme are significantly lower in F18 ETEC-resistant animals, and Chinese hamster ovary tissue culture cells transfected with the FUT1 gene coding threonine at amino acid 103 showed reduced enzyme activity.18 A test for F18 receptor genotyping has been developed and worldwide license for the test is held by the Pig Improvement Company (Franklin, Kentucky).
Methods of diagnosis and characterization of ETEC
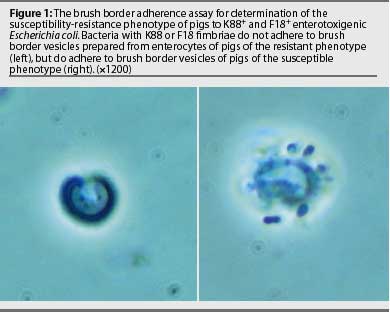
 At necropsy, pigs infected with ETEC may exhibit no observable lesions other than those associated with dehydration. However, some may exhibit mild to moderate intestinal hyperemia.19 Histologic examination of the small intestine reveals bacilli adherent multifocally or diffusely on the villous surface (Figure 2). Gram staining of impression smears from the ileum of most clinically infected pigs reveals large numbers of gram-negative bacilli, typically with few other organisms present20 and large numbers of E coli can be cultured from those tissues. Enterotoxigenic E coli cultured from infected neonatal pigs may be either hemolytic or non-hemolytic, but only hemolytic ETEC colonize weaned pigs.1,4
At necropsy, pigs infected with ETEC may exhibit no observable lesions other than those associated with dehydration. However, some may exhibit mild to moderate intestinal hyperemia.19 Histologic examination of the small intestine reveals bacilli adherent multifocally or diffusely on the villous surface (Figure 2). Gram staining of impression smears from the ileum of most clinically infected pigs reveals large numbers of gram-negative bacilli, typically with few other organisms present20 and large numbers of E coli can be cultured from those tissues. Enterotoxigenic E coli cultured from infected neonatal pigs may be either hemolytic or non-hemolytic, but only hemolytic ETEC colonize weaned pigs.1,4
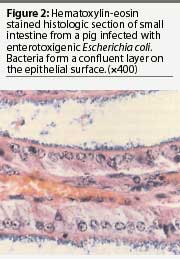
 Confirmation that E coli isolates are pathogenic strains may be by phenotypic or genotypic analysis. Phenotypic analysis is best performed by assessment for production of adhesive fimbriae, either by enzyme-linked immunosorbent assay (ELISA) or by indirect immunofluorescence assay (IFA).21 Monoclonal antibodies for each type of fimbriae are available for these tests. The ELISA test requires culture of the organisms from infected tissues. The IFA may be performed either on impression smears or frozen sections of infected intestine or on smears prepared from cultures of the bacteria (Figure 3).20, 21 A potential pitfall in using organisms cultured from infected tissue is that cultured organisms may not always express fimbriae. In vitro expression is particularly a problem with 987P and F18ab fimbriae.4,21 In addition, heavily encapsulated organisms, most frequently observed with K99 strains, may yield false-negative ELISA results.21
Confirmation that E coli isolates are pathogenic strains may be by phenotypic or genotypic analysis. Phenotypic analysis is best performed by assessment for production of adhesive fimbriae, either by enzyme-linked immunosorbent assay (ELISA) or by indirect immunofluorescence assay (IFA).21 Monoclonal antibodies for each type of fimbriae are available for these tests. The ELISA test requires culture of the organisms from infected tissues. The IFA may be performed either on impression smears or frozen sections of infected intestine or on smears prepared from cultures of the bacteria (Figure 3).20, 21 A potential pitfall in using organisms cultured from infected tissue is that cultured organisms may not always express fimbriae. In vitro expression is particularly a problem with 987P and F18ab fimbriae.4,21 In addition, heavily encapsulated organisms, most frequently observed with K99 strains, may yield false-negative ELISA results.21
Genotypic analysis by multiplex polymerase chain reaction (PCR; Figure 4 ), identifies genes for virulence factors, including fimbriae K88, K99, 987P, F18, and F41.22 It also identifies genes for toxins LT, STa, STb, and Stx2e. Testing by PCR circumvents difficulties associated with expression of genes under laboratory conditions and cumbersome assays required for identification of enterotoxins. However, the multiplex PCR test is not without its own pitfalls. Results are subject to interpretation. Superfluous bands may co-migrate with those representing genes of virulence factors. (D. Francis, unpublished data, 2002). Presence of a gene or gene fragment evidenced by a band on the agarose gel does not necessarily indicate that the gene is expressed as a functional virulence factor. Several state-supported veterinary diagnostic laboratories perform multiplex PCR tests for the virulence genes of diarrheo-genic E coli from pigs.
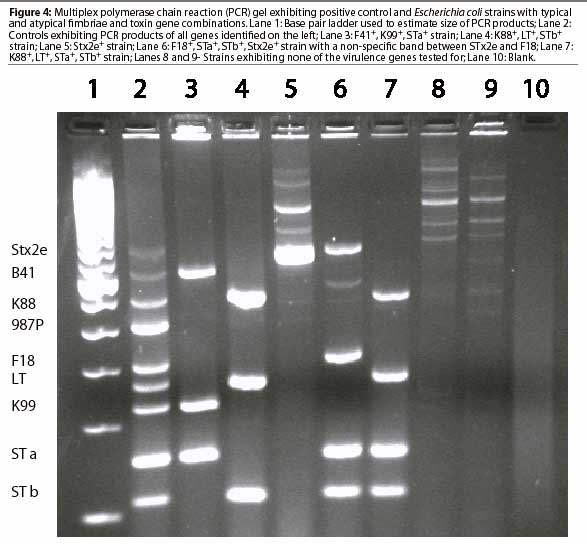
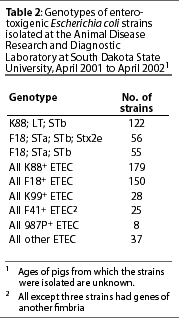 Relative prevalence of E coli strains exhibiting various genotypes in specimens submitted to the South Dakota State University Animal Disease Research and Diagnostic Laboratory from April 2001 to April 2002 is shown in Table 2. More than half of all ETEC strains isolated from pigs with diarrhea displayed one of three gene patterns: K88, LT, and STb; F18, STa, STb, and Stx2e; or F18, STa, and STb. Most strains not displaying one of these patterns either lacked one or more of the genes or had additional genes for virulence factors. When multiple E coli isolates were tested from a single herd outbreak, it was not uncommon for some isolates to display a typical virulence gene pattern, while others were missing one of the genes. The clustering of isolates missing genes with isolates having a full complement of genes may suggest that loss of virulence genes is not uncommon in ETEC. Whether that loss occurs during infection or during laboratory culture is unknown. Genes contained within bacterial plasmids may readily be disseminated through a bacterial population. As many of the virulence factor genes of ETEC are plasmid borne, the presence of atypical combinations of genes in ETEC strains is not surprising. The low incidence of isolation of each atypical combination suggests that the extra genes add little if any survival advantage.
Relative prevalence of E coli strains exhibiting various genotypes in specimens submitted to the South Dakota State University Animal Disease Research and Diagnostic Laboratory from April 2001 to April 2002 is shown in Table 2. More than half of all ETEC strains isolated from pigs with diarrhea displayed one of three gene patterns: K88, LT, and STb; F18, STa, STb, and Stx2e; or F18, STa, and STb. Most strains not displaying one of these patterns either lacked one or more of the genes or had additional genes for virulence factors. When multiple E coli isolates were tested from a single herd outbreak, it was not uncommon for some isolates to display a typical virulence gene pattern, while others were missing one of the genes. The clustering of isolates missing genes with isolates having a full complement of genes may suggest that loss of virulence genes is not uncommon in ETEC. Whether that loss occurs during infection or during laboratory culture is unknown. Genes contained within bacterial plasmids may readily be disseminated through a bacterial population. As many of the virulence factor genes of ETEC are plasmid borne, the presence of atypical combinations of genes in ETEC strains is not surprising. The low incidence of isolation of each atypical combination suggests that the extra genes add little if any survival advantage.
References – refereed
1. Wilson RA, Francis DH. 1986. Fimbriae and enterotoxins associated with E coli serogroups isolated from clinical cases of porcine colibacillosis. Am J Vet Res. 1986;47:213-217.
2. Casey TA, Nagy B, Moon HW. Pathogenicity of porcine enterotoxigenic Escherichia coli that do not express K88, K99, F41, or 987P adhesins. Am J Vet Res. 1992;53:1488-1492.
3. Westerman RB, Mills KW, Phillips RM, Fortner GW, Greenwood JM. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 1988;26:149-150.
4. Imberechts H, Bertschinger HU, Nagy B, Deprez P, Pohl P. Fimbrial colonization factors F18ab and F18 ac of Escherichia coli isolated from pigs with postweaning diarrhea and edema disease. Adv Exper Med Biol. 1997;412:175-183.
5. Francis DH, Grange PA, Zeman DH, Baker DR, Sun R, Erickson AK. Expression of mucin-type glycoprotein K88 receptors strongly correlates with piglet susceptibility to K88+ enterotoxigenic Escherichia coli, but adhesion of this bacterium to brush borders does not. Infect Immun. 1998;66:4050-4055.
6. Choi C, Cho W-S, Chung H-K, Jung T, Kim J, Chae C. Prevalence of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 (EAST1) gene in isolates in weaned pigs with diarrhea and/or edema disease. Vet Microbiol. 2001;81:65-71.
7. Clugston RE, Nielsen NO, Smith DLT. Experimental edema disease of swine (E.coli enterotoxemia). III Pathology and pathogenesis. Can J Comp Med. 1974;38:34-43.
8. Kurtz HJ, Bergeland ME, Barnes DM. Pathologic changes in edema disease of swine. Am J Vet Res. 1969;30:791-806.
9. Runnels PL, Moon HW, Schneider RA. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980;28:298-300.
10. Dean EA. Comparison of receptors for 987P pili of enterotoxigenic Escherichia coli in the small intestines of neonatal and older pigs. Infect Immun. 1990;58:4030-4035.
11. Erickson AK, Willgohs JA, McFarland SY, Benfield DA, Francis DH. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these binding glycoproteins with the adhesive porcine phenotype. Infect Immun. 1992;60:983-988.
12. Al-Majali AM, Robinson JP, Asem EK, Lamar C, Freeman MJ, Saeed AM. Age-dependent variation in the density and affinity of Escherichia coli heat-stable enterotoxin receptors in mice. Adv Exp Med Biol. 1999;473:137-145.
13. Grange PA, Erickson AK, Levery SB, Francis DH. Identification of an intestinal neutral glycosphingolipid as a phenotype-specific receptor for the K88ad fimbrial adhesin of Escherichia coli. Infect Immun. 1999;67:165-172.
14. Nagy B, Casey TA, Whipp SC, Moon HW. Susceptibility of porcine intestine to pilus-mediated adhesion by some isolates of piliated enterotoxigenic Escherichia coli increases with age. Infect Immun. 1992;60:1285-1294.
15. Baker DR, Billey LO, Francis DH. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet Microbiol. 1997;54:123-132.
16. Peelman LJ. Genetic investigation of the resistance mechanisms of the pig against diarrhea caused by E. coli. Verhandelingen-Koninklijke Academie voor Geneeskunde Belgie. 1999;61:489-515.
17. Meijerink E, Fries R, Vogeli P, Masabandi J, Eigger G, Stricker C, Neuenschwander S, Bertschinger HU, Stranzinger G. Two alpha (1,2) fucosyltransferase genes on porcine chromosome 6q11 are closely linked to the blood group inhibitor (S) and Escherichia coli F18 receptor (ECF18) loci. Mamm Genome. 1997;8:736-741.
18. Meijerink E, Neuenschwander S, Fries R, Dinter A, Bertschinger HU, Stranzinger G, Vogeli P. A DNA polymorphism influencing alpha (1,2) fucosyltransferase activity of the pig FUT1 enzyme determines susceptibility of small intestinal epithelium to Escherichia coli F18 adhesion. Immunogenetics 2000;52:129-136.
19. Moxley RA, Duhamel GE. Comparative pathogenesis of bacterial enteric diseases of swine. Adv Exp Med Biol. 1999;473:83-101.
20. Francis DH. Use of immunofluorescence, gram staining, histologic examination, and seroagglutina-tion in the diagnosis of porcine colibacillosis. Am J Vet Res. 1983;44:1884-1888.
21. Mullaney CD, Francis DH, and Willgohs JA. Comparison of seroagglutination, ELISA, and indirect fluorescent antibody staining for the detection of K99, K88, and 987P pilus antigens of Escherichia coli. J Vet Diagn Invest. 1991;3:115-118.
References – non refereed
22. Casey TA, Bosworth BT. Diagnosis of E. coli in swine using multiplex PCR. 97th Gen Meet Amer Soc Microbiol. Miami, Florida. 1997;116.
This page last updated .
