Winkelman NL. 2002;3:107-111 Lincomycin-medicated feed for the c
Original research
Peer reviewed
Lincomycin-medicated feed for the control of porcine proliferative enteropathy (ileitis) in swine
Nathan L. Winkelman, DVM; John P. Crane, BSc; Gregory D. Elfring,
MS; D. Dal Kratzer, PhD; David M. Meeuwse, BS;
Ken J. Dame, BS; Susan L. Buckham, MS; Connie J. Gebhart, PhD
Winkelman NL, Crane JP, Elfring GD, et al. Lincomycin-medicated feed for the control of porcine proliferative enteropathy (ileitis) in swine. J Swine Health Prod. 2002;10(3):107-111. Also available as a PDF.
NLW: Swine Services Unlimited Inc, 10 E 6th Street, Morris, MN 56267; JPC, GDE, DDK, DMM, KJD, SLB: Pharmacia Animal Health, Division of Pharmacia Corp, Kalamazoo, MI 49001; CJG: Veterinary Pathobiology, University of Minnesota, St Paul, MN 55108. Corresponding author: John P. Crane, Pharmacia Corp, 7000 Portage Rd, Kalamazoo, MI 49001; Tel: 616-833-2646; E-mail: john.p.crane@pharmacia.com.
Summary
Objective: To evaluate the efficacy of lincomycin (Lincomix 20 Feed Medication; Pharmacia Animal Health, Kalamazoo, Michigan) at 44 and 110 ppm administered in feed for control of porcine proliferative enteropathy (PPE) in swine challenged with mucosal homogenate.
Methods: A total of 312 commercial crossbred pigs at two sites were randomly assigned to receive lincomycin in feed at either 44 or 110 ppm, or no treatment. On 2 consecutive days, each pig was inoculated orally with Lawsonia intracellularis in the form of porcine intestinal mucosal homogenate. Animals were observed until clinical signs of disease were seen, then pigs assignedto treatment groups received lincomycin in the feed daily for 21 days. Control animals received no treatment. Clinical observations and performance were assessed throughout the study. Necropsies were conducted at the end of the study or at death.
Results: The L intracellularis challenge produced a high incidence of clinical disease. The incidence of diarrhea and scores for abnormal clinical impression were lower and ADG and feed conversion efficiency were better in groups treated with either dose of lincomycin than in untreated controls. Mortality was lower in the group treated with lincomycin at 110 ppm than in the untreated controls.
Implications: Under the conditions of this study, lincomycin administered in feed at 44 and 110 ppm for 21 consecutive days was effective in controlling clinical signs of PPE, and at 110 ppm also reduced mortality associated with PPE.
Keywords: swine, porcine proliferative enteropathy,Lawsonia intracellularis, lincomycin, feed medication
Received: March 16, 2001
Accepted: December 10, 2001
Porcine proliferative enteropathy (PPE), also known as ileitis, is an enteric disease of pigs worldwide.1-3 It is characterized by thickening of the mucosaof the small intestine and occasionally the large intestine. Pathognomic lesions consist of proliferative immature crypt cells in the intestinal epithelium, with free-floating bacteria in the apical cytoplasm.The causative agent is Lawsonia intracellularis,1-3 but development of PPE requires the involvement of other enteric bacteria. This was demonstrated by McOrist et al,4 who showed that gnotobiotic pigs are resistant to challenge and infection by L intracell-ularis unless pre-dosed with a minimal inoculum of normal gastrointestinal microflora. A variety of Campylobacter species have been isolated from proliferative lesions. However, oral inoculation of these bacteria has never reproduced the disease, suggesting that they are secondary agents which may contribute to the disease.1,4-6
The use of polymerase chain reaction (PCR) and immunological detection methods has shown that strains of L intracell-ularis isolated from geographically distinct worldwide locations are practically indistinguishable.2 Isolates from different geographical locations also have similar fastidious growth characteristics.2,7 Pathogenicity of both US and European strains has been confirmed in model studies.1,2,5
Porcine proliferative enteropathy occurs in a chronic form, comprising both necrotic enteritis and porcine intestinal adenopathy (PIA), and an acute hemorrhagic form known as porcine hemorrhagic enteropathy. A subclinical form also exists.1,8,9
Chronic PPE, the most common form of the disease, occurs mainly in growing pigs 6 to 20 weeks of age,1,3 and manifests primarily as a transient reduction in the rate of weight gain. Inappetence and diarrhea are also occasionally observed. Commonly, clinical signs are not readily apparent.1,8,9 When diarrhea occurs, it is generally moderate, with loose stools of normal to gray-brown color and no visible blood. Morbidity is usually 5 to 20% in affected herds.1,3
The acute form of the disease occurs more commonly in young adults (4 to 12 months old) and is manifested by acute anemia, hemorrhagic diarrhea, and sudden death. Up to 50% of affected animals may die.1,3
In vivo hamster challenge models and in vitro testing of L intracellularis in host cell tissue culture systems have been used in an attempt to predict the efficacy of various antibiotics in treatment of PPE.3,10 However, it is difficult to correlate the results of these studies to field efficacy. Challenge models have been developed in swine. A swine challenge model was employed in a study to determine appropriate in-feed doses of lincomycin for a prevention and control program.11 Daily treatment with lincomycin at either 44 ppm or 110 ppm was initiated 4 days before challenge. Both doses were found to be effective in reducing mortality and improving production parameters compared to no medication. Although prevention-control, in-feed programs are used in the field, treatment is more commonly initiated when clinical signs are already present in the herd. This study was designed to evaluate the efficacy of in-feed lincomycin in controlling PPE (ie, reducing mortality, reducing incidence and severity of clinical signs, and improving production parameters) when treatment was not initiated until inoculated animals were showing clinical signs of PPE.
Materials and methods
Study animals
A total of 312 commercial crossbred pigs were included in the study. Pigs of known health status were purchased from local farms that used segregated early weaning management. Healthy pigs weighing between 6.8 and 13.6 kg were acceptable for inclusion. At the time the pigs were purchased, the source farms had no history of clinical disease due to L intracellularis, Brachyspira hyodysenteriae, B pilosicoli, Salmonella serovar Choleraesuis, Actinobacillus pleuropneumoniae, transmissible gastroenteritis virus, or porcine reproductive and respiratory syndrome (PRRS) virus. The source herds were screened for PPE by indirect fluorescent antibody (IFA) assay (reciprocaltiter of 30 was considered positive) and for PRRS virus by ELISA (HerdChek PRRS ELISA; IDEXX Laboratories, Westbrook, Maine) (a sample:positive ratio >=0.4 was considered positive).
Housing and feeding
The study was conducted at Sites A and B, two locations in Minnesota remote from other swine facilities.
Site A. One hundred and eighty pigs were housed in two adjacent modern nursery barns, each with two rows of eight pens (3.0 m2) separated by an alley. Pens had perforated plastic grating floors, and each pen provided one five-hole dry-feed feeder and two nipple waterers. In each barn, pigs were housed in 15 pens in groups of six. Solid plastic partitions between pens prevented pig-to-pig contact and feed transfer. The buildings were environmentally controlled using negative-pressure mechanical ventilation with integrated heater and fan controllers. Barn and ambient temperatures were measured continuously by computer.
Site B. One hundred and thirty two pigs were housed in a single barn, which was a renovated, wooden pig nursery building containing two rows of five and six pens (3.6 m2) separated by an alley. Pens had vinyl-covered expanded metal floors, and each pen provided one six-hole dry-feed feeder and two nipple waterers. Solid vinyl planks fitted together prevented pig-to-pig contact and feed transfer between pens. Pigs were housed in groups of 12. Heat was provided by a thermostatically controlled gas-fueled furnace. Ventilation consisted of an exhaust fan for exit air and an inlet mixing tube which allowed outside air to be blended with inside air and circulated over the pens. Environment monitors automatically recorded the building temperature.
Treatment assignments
At each site, pigs were randomly allocated to pens and pens to treatment by a computer-generated plan using PROC PLAN of the SAS System.12 Pens of the same block were located as close as possible to each other.
Site A. The experimental design was a randomized complete block design. Pigs were grouped in blocks by weight before challenge. A pen of six pigs was the experimental unit. Treatment groups received one of two dosages of lincomycin (Lincomix 20 Feed Medication; Pharmacia Animal Health, Kalamazoo, Michigan) administered in feed. A complete block contained three pens: one for each treatment group (44 and 110 ppm lincomycin), and one for the nonmedicated control group. There were ten replicates of each treatment and the control group.
Site B. The experimental design was a randomized incomplete block design. Pigs were grouped in blocks by weight before challenge. A pen of 12 pigs was the experimental unit. Treatment groups received one of two dosages of lincomycin (Lincomix 20 Feed Medication; Pharmacia Animal Health) administered in feed. A complete block contained three pens: one for each treatment group (44 and 110 ppm lincomycin) and one for the nonmedicated control group. One block was incomplete because there was one less pen for the 110 ppm group, ie, there were four replicates of the 44 ppm treatment group and the nonmedicated control group, and three replicates of the 110 ppm treatment group.
Inocula
The inoculum used in this study was pooled intestinal mucosal homogenate derived from previously challenged pigs manifesting gross pathology of PPE. To obtain the homogenate, mucosa was scraped from small intestines affected with moderate to severe PIA and (or) necrotic PPE lesions, and suspended at a 1:1 ratio in sucrose-phosphate-glutamate (SPG) buffer, pH 7.0, which contained sucrose (0.218 mol per L), monobasic potassium phosphate (KH2PO4; 0.0038 mol per L), dipotassium phosphate (K2HPO4; 0.0072 mol per L), and L-glutamic acid (0.0047 mol per L). The source for all reagents was Sigma (St Louis, Missouri). The mucosal suspension was homogenized in a blender. Homogenates were stored at -70°C until used, and thawed and stored on ice on days of use. Samples were submitted on ice to the Department of Veterinary Pathobio-logy, University of Minnesota, for enumeration of L intracellularis by immunoperoxidase staining, and to confirm absence of the following potentially confounding enteric pathogens (methods in parentheses): Brachyspira species (dark field microscopy), Salmonella Choleraesuis (culture on sheep blood agar, MacConkey agar, XLD agar, Brilliant Green agar), and [beta]-hemolytic Escherichia coli (culture on sheep blood agar, MacConkey agar). All pigs at both sites were inoculated before assay results were available.
At each site, a total infective dose of 108 to 109 L intracellularis was estimated (on the basis of findings in previous studies) and administered by gavage to each pig in two equal divided doses of approximately 15 to 30 mL per dose, 24 hours apart.
Records and observations
The study was conducted according to both US13 and European14 Good Clinical Practice guidelines. All personnel who assessed and recorded clinical signs and who performed and recorded performance measures were masked to treatment.
Evaluation of efficacy was based a test-control comparison of percent mortality, percent of pigs with diarrhea, percent of pigs with abnormal clinical impression scores, and growth performance parameters, including average daily gain (ADG), feed intake, and feed conversion efficiency (FCE). Mortality, diarrhea, and clinical impression scores were recorded every day from inoculation (Day 0) until the day treatment began, then every other day until study termination.
Diarrhea scores were assigned as follows: 1 = no diarrhea; 2 = semi-solid stool, no blood; 3 = watery stool, runs through the floor slats, no blood; 4 = blood-tinged feces, loose or formed; 5 = profuse diarrhea with blood or very dark tarry feces.
Clinical impression scores were obtained from the sum of the pig demeanor score and the abdominal appearance score, divided by two. Pig demeanor scores were recorded as follows: 1 = normal; 2 = slightly to moderately depressed, listless, will stand; and 3 = severely depressed, recumbent, unwilling to stand. Abdominal appearance scores were recorded as follows: 1 = normal; 2 = moderately gaunt; 3 = severely gaunt.
Feed consumption and body weights were recorded weekly. Feed was weighed daily and added manually to individual feeders. Uneaten feed was weighed weekly to permit calculation of weekly pen feed consumption. Pen feed consumption was correctedfor mortality by calculating consumption by pig-day. One pig-day was each day one pig was alive in the pen.
Necropsies were performed on all pigs by the investigator either
at death or at study termination (one day after the end of the
21-day treatment period). Gross pathological evaluation of the
intestines was made by the investigator. Ileal segments from all
pigs were submitted in buffered formalin to the Department of
Veterinary Pathobiology, University of Minnesota, for histopathological
testing using an immunoperoxidase staining technique incorporating
monoclonal antibody to
L intracellularis.
Treatment initiation
At Site A, treatment was to be initiated when >=10% of pigs in each barn had cumulatively manifested either watery diarrhea (score >=3) or abnormal clinical impressionscores (>=2). At Site B, treatment was to be initiated when >=20% of the pigs had cumulatively manifested either abnormal fecal scores (>=2) or abnormal clinical impression scores (>=2), with at least one pig per pen meeting these criteria. Until treatment began, all animals were fed the same unmedicated diet. Medication was added to this diet at 44 or 110 ppm for the two treatment groups, and unmedicated feed continued to be supplied to the control groups. Diets were manufactured in bulk and were color and letter coded to maintain masking. Samples of each batch were analyzed before the study to confirm the correct concentrations of lincomycin and freedom from other antibiotics.
Statistical design and analysis
The experimental design for both studies was a randomized block design with pen as the experimental unit. Pigs were blocked by weight to reduce the impact of weight as a potential confounding factor. Treatment efficacy was assessed by measures of pen mortality, diarrhea, clinical impression, and performance variables as defined below. Mortality was defined as the percentage of deaths in a pen due to PPE. Diarrhea was defined as the percentage of pig observations in a pen with a diarrhea score >=2 duringthe treatment period. Abnormal clinical impression was defined as the percentage of pig observations in a pen with either a pig demeanor score or an abdominal appearance score >=2 during the treatment period.
Average daily feed intake (ADFI) was calculated by dividing the total amount of feed consumed during the treatment period by the number of pig-days. Average daily gain was calculated by dividing total weight gain of the pen by the number of pig-days. Feed conversion efficiency was calculated by dividing ADG by ADFI.
Animals that died of diseases other than PPE were excluded from the analyses. To adjust feed consumption for excluded animals, the pen average for individual daily consumption was subtracted for each day the excluded pig was present in the pen.
Treatment differences in these variables were investigated using a pooled analysis of variance for the two sites, weighted on the number of pigs per pen. When necessary, variables based on pen percentages were transformed using the Freeman-Tukey arcsin to better satisfy model assumptions for the statistical analyses. Mean values and the P values for the one-sided t test ([alpha]=.05) of treatment comparisons represent the results of the pooled analysis.
Results
The source herds were serologically negative for PRRS virus and L intracellularis. Average pig weights at the beginning of the study were 9.3 kg at Site A and 10.8 kg at Site B.
Brachyspira species, Salmonella Cholerae-suis, and [beta]-hemolytic E coli were not identified in the challenge homogenates. The challenge dose of L intracellularis was 5.5×108 organisms per pig at Site A, and 1.8×109 organisms per pig at Site B.
Treatment was initiated at each site on Day 7 post challenge. At Site A, 50% of pigs in one barn and 60% of pigs in the other had cumulatively manifested grade 3 diarrhea by Day 7, with this score observed in at least one pig in 100% of pens. The pre-treatment occurrence of diarrhea was nearly equal in the nonmedicated control group (30.4% of pig-days), the 44-ppm-lincomycin group (29.7% of pig-days), and the 110-ppm-lincomycin group (28.9% of pig-days). At Site B, 61% of pigs had cumulatively manifested grade 2 diarrhea and 40% had manifested grade 3 diarrhea by Day 7, with at least grade 2 diarrhea observed in at least one pig in 100% of pens. Pre-treatment diarrhea occurred less frequently in the nonmedicated control group (15.8% of pig-days) than in the 44-ppm-lincomycin group (24.6% of pig-days) or the 110-ppm-lincomycin group (26.3% of pig-days).
A total of 40 of the 312 pigs died or were humanely euthanized during the 28-day study period. Eleven were diagnosed pre- and (or) post-mortem with diseases other than PPE. Enteritis associated with Salmonella serovars or E coli was diagnosed in five pigs, pulmonary disease in two pigs, and miscellaneous morbidities in four pigs. Data obtained from these 11 pigs were excluded from the analysis.
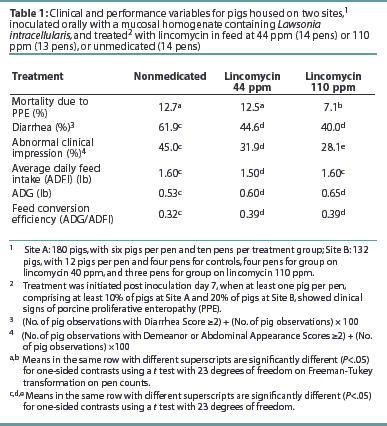
Results for the primary clinical variables (mortality due to PPE, diarrhea, and abnormal clinical impression) are presented in Table 1. Mortality was lower for the group treated with 110 ppm lincomycin (P<.05) than for unmedicated controls. Groups treated with either 44 or 110 ppm lincomycin had less occurrence of diarrhea and abnormal clinical impression (P<.05) compared to the nonmedicated controls (Table 1). Also, groups treated with either 44 or 110 ppm lincomycin had better ADG and FCE (P<.05) than the nonmedicated control group (Table 1).
Necropsy results confirmed infection with L intracellularis: greater than 50% of pigs either manifested classical PPE intestinal lesions or were positive for L intracellularis by tissue immunohistochemistry.
Discussion
In addition to mortality, the disease parameters important to the pig producer are principally performance related. The chronic form of PPE primarily causes a depression in FCE and growth,1,3 resulting in increased time to market, increased feed costs, and uneven pig weights. All of these factors increase the producer’s cost of raising pigs. In addition to such management practices as improved sanitation and reduction of stress factors, the strategic use of an in-feed antibiotic, such as lincomycin, to control the disease and help maintain uniform pig performance through the period of risk, is an important consideration.
The PPE challenge model used in this study utilizes conventional market pigs. It consistently reproduces clinical signs and pathology typical of field cases of PPE15 and has been shown to be repeatable. Performance of the model in this study was consistent with previous studies (N. Winkelman, DVM, unpublished data, 2001).
On necropsy, diseases other than PPE were diagnosed in a small minority of pigs (3.5%), principally enteritis due to either salmonellae or E coli. This multi-etiology has been observed in field cases of PPE.16,17 These pathogens were not identified in the mucosal homogenates used as inocula, and it is, therefore, most likely that the sources were the pigs themselves. Results from these animals were excluded from the analysis. As lincomycin is not active against members of the Enterobacteriaceae, it is not likely that any such underlying pathogenic etiology in the non-excluded pigs biased the results in favor of lincomycin. The incidence and severity of disease occurring at both sites (40% to 55% incidence of grade 3 diarrhea) prior to initiation of treatment was probably greater than would normally occur in the field. Nevertheless, lincomycin administered in feed at both 44 and 110 ppm was effective in reducing clinical signs and ameliorating performance deficits (ADG and FCE) due to PPE.
Results of this study and data from a previously published report11 show that in-feed regimens of lincomycin (44 and 110 ppm) approved for the control and treatment of swine dysentery, a disease that manifests clinical signs similar to those of PPE, are also effective in the treatment of PPE.
Implications
- Under the conditions of this study, lincomycin administered in feed at either 44 or 110 ppm for 21 consecutive days, beginning after the onset of clinical signs, controlled diarrhea and clinical signs associated with PPE in treated pigs compared to nonmedicat-ed controls.
- Under the conditions of this study, lincomycin administered in feed at either 44 or 110 ppm for 21 consecutive days, beginning after the onset of clinical signs, improved ADG and FCE in treated pigs compared to nonmedicated controls.
- Under the conditions of this study, lincomycin at 110 ppm reduced mortality due to PPE in treated pigs compared to nonmedicated controls.
References – refereed
1. McOrist S, Gebhart C. Porcine proliferative enteropathies. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:521-534.
2. Knittel JP, Larson DI, Harris DL, Roof MB, McOrist S. United States isolates of Lawsonia intracellularis from porcine proliferative enteropathy resemble European isolates. Swine Health Prod. 1996;4:119-122.
3. McOrist S, Mackie RA, Lawson GHK. Antimicrobial susceptibility of ileal symbiont intracellularis isolated from pigs with proliferative enteropathy. J Clin Microbiol. 1995;33:1314-1317.
4. McOrist S, Mackie RA, Neef N, Aitken I, Lawson GHK. Synergism of ileal symbiont intracellularis and gut bacteria in the reproduction of porcine proliferative enteropathy. Vet Rec. 1994;134:331-332.
5. McOrist S, Jasni S, Mackie RA, MacIntyre N, Neef N, Lawson GHK. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Imm. 1993;61:4286-4292.
6. Cooper DM, Gebhart, CJ. Comparative aspects of proliferative enteritis. JAVMA. 1998;212:1446-1451.
7. Collins AM, Swift I, Monckton RP. Replication of Australian porcine isolates of ileal symbiont intracellularis in tissue culture. Vet Microbiol. 1996;49:249-255.
8. Ward GE, Winkelman NL. Diagnosing, treating, and controlling proliferative enteritis in swine. Vet Med. 1990;85:312-319.
9. Ward GE, Winkelman NL. Recognizing the three forms of proliferative enteritis in swine. Vet Med. 1990;85:197.
12. SAS/STAT User’s Guide, Version 6. Vol 2. 4th ed. Cary, North Carolina: SAS Institute Inc; 1989.
13. Guidance for Industry Number 58: Good Target Animal Study Practices: Clinical Investigators and Monitors. Rockville, Maryland. US Dept of Health and Human Services, Food and Drug Administration, Center for Veterinary Medicine; May 1997.
14. Good Clinical Practice for the Conduct of Clinical Trials for Veterinary Medicinal Products. GCPV. The EU Note for Guidance. Brussels, Belgium: FEDESA; July 1995.
15. Marsteller T, Winkelman N, Gebhart CJ, Armbruster G, Weldon W, Muller R, Weatherford J, Symanowski J. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet Ther. 2001;2(1):51-60.
16. Thomson JR, Smith WJ, Murray BP. Investigations into field cases of porcine colitis with particular reference to infections with Serpulina pilosicoli. Vet Rec. 1998;142:235-239.
17. Møller K, Jensen TK, Jorsal SE, Leser TD, Carstensen B. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-haemolytic intestinal spirochaetes, Salmonella enterica, and haemolytic Escherichia coli from swine herds with and without diarrhoea among growing pigs. Vet Microbiol. 1998;62:59-72.
References – non refereed
10. Gebhart CJ, McOrist S. A hamster challenge model for evaluating effectiveness of chlortetracycline in prevention/control of porcine proliferative enteropathy. Proc AASP. 1995;93-99.
11. Winkelman N. Dose determination trial for lincomycin in the control of porcine proliferative enteropathy. Proc Allen D. Leman Swine Conf Res Abstr. 1998:27.