Straw B. 2002;2:75-79 Factors associated with death due to hemor
Original research
Peer reviewed
Factors associated with death due to hemorrhagic bowel syndrome in two large commercial swine farms
Barbara Straw, DVM, PhD; Catherine Dewey, DVM, PhD; James Kober, DVM; Steven C. Henry, DVM, Dipl ABVP
BS: Dept of Large Animal Clinical Sciences, Michigan State University, E Lansing, MI 48824; CD: Dept of Population Medicine, University of Guelph, Guelph, Ontario N1G 2W1; JK: Swine Veterinary Services of Michigan, Zeeland, MI 49423; SCH: Abilene Animal Clinic, Abilene, KS 67410.
Straw B, Dewey C, Kober J, et al. Factors associated with death due to hemorrhagic bowel syndrome in two large commercial swine farms. J Swine Health Prod. 2002;10(2):75-79. Also available as a PDF.
Summary
Objective: To investigate the roles of season, gender, animal density, concurrent disease, and antibiotic feeding regimen on the occurrence of hemorrhagic bowel syndrome (HBS).
Methods: Retrospective analysis of finishing records from two large commercial swine herds.
Results: Gender, animal density, and concurrentdisease were not associated with death due to HBS. Rates of death due to HBS were higher for pigs finished during the summer than for those finished in the winter. Rates of death due to HBS were lower in pigs fed rations containing bacitracin methylene disalicylate or chlortetracycline than in pigs on rations containing virginiamycin or no antibiotic.
Implications: A definitive cause of HBS is still lacking; however, these results lend support to a non-infectious etiology involving intestinal volvulus. To further investigate whether HBS and volvulus are two separate syndromes, veterinarians and farm personnel trained to perform necropsies should palpate the mesenteric root before opening the abdomen.
Keywords: swine, hemorrhagic
bowel syndrome,volvulus, antibiotics, season
swine, hemorrhagic
bowel syndrome,volvulus, antibiotics, season
Received: August 15, 2001
Accepted: November 26, 2001
Hemorrhagic bowel syndrome (HBS) has been described since 1959.1 Between 1959 and 1970, HBS accounted for only about 2 to 5% of the yearly finishing pig mortality with occasionalsporadic outbreaks reported in which 10 to 20% mortality occurred.1 The condition was seen primarily in Europe with an occasional report in the United States.2,3 Hemorrhagic bowel syndrome has taken on more importance and prominence in the last decade. It primarily affects rapidly growing pigs between 4 and 6 months of age (70 to 120 kg). The size and otherwise excellent health of the affected animals makes this condition of particular economic importance.
There is considerable ambiguity in the clinicopathological definition of HBS. The term "hemorrhagic bowel syndrome" has been applied when finishing swine die suddenly without premonitory evidence of diarrhea or other clinical signs, and on post mortem examination of a recently dead animal, there is marked pallor of the skin and pronounced distention of the abdomen. The small intestine is thin-walled and filled with either clotted or unclotted blood. The large intestine contains tarry fecal material, but lesions suggestive of gastriculceration, necroproliferative enteritis, salmonellosis, swine dysentery, or other identifiable disease processes are absent. Histologically, there may be no significant lesions. However, in some cases, autolysis of villous epithelium, diffuse congestion of blood vessels in the lamina propria and submucosa, occasional focal to diffuse hemorrhage into the lamina propria, and diffuse infiltration of eosinophils and plasma cells into the lamina propria may be observed.4 Volvulus of the intestine is variably identified. Smith and Shanks5 noted that intestinal volvulus was sometimes present at post mortem examination and suggested that this was the result of agonal spasm. Rowland and Lawson6 also drew attention to volvulus and suggested that as volvulus was often difficult to detect, it might be more common than previously recognized. In a Swiss report7 of 436 cases, intestinal volvulus was confirmed in 56% of those examined in the first 8 years of the study, and in 80% of cases in the next 2 years, when more careful examination was conducted.
The question has arisen whether HBS and volvulus are equivalent. Much of the uncertainty seems rooted in the difference in interpretation of the definition of HBS as portrayed in the literature published by European (primarily UK) workers compared to publications emanating from North America. In the early 1970s, the term "HBS" appears to have been discontinued by UK investigators on the basis of their belief that in the majority of cases, volvulus was present but undiagnosed. This interpretation was not then, and is not now, accepted in North America. Many pathologists, laboratory diagnosticians, and practitioners in North America believe that there is an ill-defined dividing line between HBS and volvulus at opposite ends of a continuum. One hypothesis is that inciting events induce overgrowth and (or) alteration of normal flora in the GI tract with consequent over-production of toxic substances and damaging compounds. This is well documented in ruminants, where various risk factors contribute to overgrowth of normal intestinal flora, with sequelae such as acidosis, endotoxemia, enterotoxemia, and death.8 Thus, HBS may be a disease caused by normal, usually non-pathogenic inhabitants of the GI tract that proliferate under certain conditions. In this paper, the term "HBS" is used when the classic gross and post-mortem appearance occurs, but volvulus either has not been identified or has not been looked for. When the classic signs are present, and in addition volvulus has clearly been identified, then the term "volvulus" is used.
Death from HBS is attributed to profound circulatory impairment secondary to severe intestinal distention and hemorrhage, and also possibly an associated volvulus. The inciting agent(s) for these pathologic changes has not been identified; however, several mechanisms have been proposed, including disturbance in feed intake, physical trauma, infection, and immediate hypersensitivity. The objectives of this study were to employ retrospective analysis of data from two commercial swine operations to investigate the roles of season, gender,animal density, concurrent disease, and antibiotic feeding regimen on the occurrenceof HBS.
Materials and methods
Farm 1
A retrospective analysis of finishing barn records from pigs
in a multi-site wean-to-finishcommercial operation was performed,
using records from five of the 17 finishing site locations. This
provided data from 24 groups, each composed of approximately 1000
pigs. Each site had two to four 1000-head wean-to-finish barns
with total concrete slats, curtain sides, and tunnel ventilation
(Hog Slat, Newton Grove, North Carolina). Barns contained either
only barrows or only gilts to facilitate split-sex phase feeding.
Groups of pigs were moved into the barns at weaning
(approximately18 days of age) and stayed for 26 weeks (until marketed).
Feed and pigs were supplied from a common source. Pigs were of
PIC genetics.
Feed contained an antibiotic or a combination of several different antibiotics during the grow-finish period, for growth promotion or as dictated by occurrence of disease. Between days 100 and 175 after arrival, feed for most groups sequentially contained two or three different antibiotics. The usual practice was to rotate different antibiotics in the feed every 2 to 3 weeks, except in the case of tylosin at 100 g per ton, which was fed for 5 to 7 days at a time.
Pigs were allotted 0.7 m2 of pen space. Feed was delivered to a wet-dry feeder. Water was provided through a swinging nipple waterer until pigs weighed 18 kg, then waterto the nipple was shut off and water was supplied through the feeder.
At four of the five sites, records were available for groups of pigs that entered the barns in the winter (November 14 to February 6) and also for groups of pigs that entered the following summer (May 15 to August 14). The fifth site had records only from pigs that entered in the spring (March 20 to 27). At each site, data recorded by the farm managers included a current inventory of pigs, updated each day that pigs died or were transferred or sold; mortality records, including date and reason for death; dates of feed deliveries specifying ration and antibiotic; and summary information including market weight, feed conversion efficiency, average daily feed intake, and average daily gain. Diagnosis of the cause of death was made by the farm manager using criteria supplied by the farm owner, eg, HBS was listed as the cause of sudden death for a previously healthy, well-grown pig with a pale carcass and bloated abdomen. Frequently, a cursory necropsy was performed to confirm the presence of a thin-walled small intestine filled with gas and either clotted or unclotted blood.
Calculations. Weekly death loss due to HBS or to any other cause by number of weeks in the barn was calculated for each group of pigs that had been started either in the summer or the winter. Weekly death loss was calculated as the number of deaths that week divided by the current inventory of pigs in the barn, multiplied by 100. Death loss by week was plotted for pigs started in the winter and in the summer. Data for HBS death loss was smoothed by calculating each data point as the median of that point and the one on each side of it. Data from pigs started in the spring was not examinedsince there were only two barns in this group.
To examine the relationship between pig density and HBS death rate, rates within each barn were compared between weeks 25 and 26 (when pigs were being removed from the barn and populations were reduced by approximately 25 to 50%) and weeks 23 and 24 (before any pigs were sold). During each 2-week time frame, the average barn population and the total number of HBS deaths were used to calculate HBS mortality rate.
For pigs that entered the barns in winter and summer, the risk rate of dying from HBS was calculated for each of six different in-feed antibiotics or unmedicated feed. A true rate was calculated for each antimicrob-ial product used within each barn. The denominatorwas the number of pigs consumingthe product multiplied by the number of days the product was used. The numerator was the number of pigs that died of HBS during that time period. Therefore, the unit of analysis was the barn.9 The calculated true rate was a close approximation to a risk rate because the rate was extremely low. The rate for each antibiotic administered in the ration of at least 800 pigs for at least 50 days was determined. The average rate of pigs dying from HBS was determined by product and by season.
Statistical methods. Descriptive statistics were used to describe the extent and temporal occurrence of deaths due to HBS and other causes, and to calculate rates of mortality due to HBS for pigs consuming rations containing various antibiotics.
Pearson’s correlation coefficient was used to examine the association between death loss from HBS and other causes. Chi-square analysis was used to compare death loss due to HBS and other causes between genders,and within each barn when it was full and after 25 to 50% of the pigs had been removed. On any site, when both gilts and barrows were weaned and placed in the finishing barns within a day or two of each other, mortality due to HBS and due to any cause were compared between genders within the same site.
Rates of mortality due to HBS were compared between seasons within barns and within week after fill, using paired t tests. Paired t test was also used to compare mortality rates within each barn when it was full and after some pigs had been removed.
True rates were used to compare death rates of pigs consuming different antibiotics. Comparisons between rates of mortality due to HBS by product were determined using linear regression and regressing the HBS death rate on the product and season. The results were expressed as the number (x 10-5) of pigs dying of HBS per day, per 1000 pigs at risk during days 100 to 175 after entering the wean-to-finish barn.
Farm 2
Causes of finishing pig deaths were available for analysis in a large integrated swine unit that produced over one million pigs per year. This farm was managed using three-site production. Pigs were in the finishing barns for 16 weeks. Most barns were similar to those on Farm 1. When this data was collected, the farm was free of porcine reproductive and respiratory syndrome (PRRS) and Mycoplasma hyopneumoniae pneumonia. Pigs were of PIC genetics. Mortality data was available for 9 months of 1998 (January to September) and the entire year 1999. Causes of death were recorded with age, gender, and time of year. Veterinarians performed necropsies on all pigs that died. The necropsy procedure for finishing swine that died suddenly, without prior evidence of diarrhea or other clinical signs, and had marked pallor of the skin and pronounced distention of the abdomen, was modified to include making a small incision in the abdominal wall and palpating the mesenteric root before opening the abdomen.
Calculations and statistical methods. The numbers of pigs that died each week from the three major causes of death were determined and plotted by week after fill. Descriptive statistics were used to summarize causes of death. The number of pigs housed in each finishing barn was not available; therefore, data could be analyzed only as relative frequencies within the entiredata set of dead pigs.
Results
Farm 1
Prevalence of HBS mortality. In five of the 24 groups of pigs, no deaths were attributed to HBS, while in the two most severelyaffected groups, HBS accounted for the deaths of 23 of 1040 pigs (2.21%) and 25 of 1115 pigs (2.24%) placed in the barns. The mean percent of pigs dying from HBS per group was 0.9% +/- 0.7% (range 0 to 2.24%). Deaths from other causes averaged 5.3% +/- 3.9% (range 0.8 to 16.6%). By Pearson’s correlation, the percent of pigs dying of HBS in a finishing group was not associated with the percent of pigs dying from other causes in that group (corr = -0.03).
Mortality by gender: A comparison of HBS mortality between genders was made in eight pairs of barns, with one gilt barn and one barrow barn in each pair. By chi-square analysis, there was no difference between genders (P>.08) in seven cases, and in the eighth case, there was a higher incidence (P=.03) in barrows (2.28%) than gilts (1.03%). The proportion of barrows dying of any cause was greater in each season than for gilts, but only significantly so in the summer (Table 1).

Mortality by season and weeks from time of fill. In
summer and in winter, HBS was not seen until after the pigs had
been in the barn 6 weeks. After that, there was a low incidence
that gradually rose in a nearly linear fashion to its highest
levels (approximately one pig per week in a 1000-head barn) between
20 and 24 weeks. The average weekly HBS mortality rate for pigs
that entered finishing barns in the winter was 0.028%, compared
to 0.051% for pigs that entered in the summer. When a paired
t test was used to examine the difference at each site
between seasons for each week after the fill, weekly HBS mortality
rate was higher in pigs started in the summer than in the winter
(P=.0004).
The weekly mortality rate for other causes of death by week within each season was more constant throughout finishing with the exception of a sharp increase during the fall (weeks 17 through 20) in the pigs that entered the facility in May and June. This mortality was caused by an outbreak of respiratory disease associated with swine influenza virus, PRRS virus, and Haemophilus parasuis. There was also an increase in recorded deaths during weeks 25 and 26 due to euthanasia of runt and cull pigs as barns were being emptied.
Mortality by number of pigs in the barn. The mean number of pigs in the barns (+/- SD) was 863 +/- 65 during weeks 23 and 24 and 507 +/- 108 during weeks 25 and 26. There was no difference (P=.11) in the HBS death rates during weeks 23 and 24 compared to the rates in weeks 25 and 26 (paired t test).
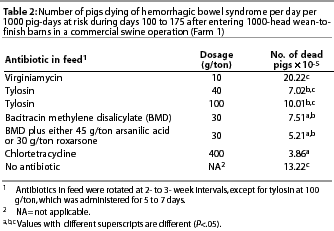
Mortality by antibiotic in ration. Antimicrobial products administered in the feed includedbacitracin methylene disalicylate (BMD), bacitracin zinc, roxarsone, arsanilic acid, tylosin, chlortetracycline, and virginiamycin (Table 2). The rate of HBS death loss was lower (P=.05) when feed contained 400 g chlortetracycline per ton, 30 g BMD per ton, or 30 g BMD plus an arsenical compound, compared to no antibiotic or 10 g virginiamycin per ton (Table 2).
Farm 2
Death loss in finishing pigs was very low throughout this complex. In 1999, of the approximately 1.2 million pigs that were finished, only 14,344 pigs died, resulting in an overall mortality rate near 1.2%. Intestinalvolvulus was the leading cause of death in 1998 (30.4% of deaths) and in 1999 (27.6% of deaths). The other two major causes of death were dermatitis-nephropathy syndrome (15.4% in 1998 and 16.3% in 1999) and gastric ulceration (13.7% in 1998 and 13.2% in 1999).
Mortality by gender. The proportion of barrows and gilts dying from intestinal volvulus did not differ in 1998 (P=.30) or 1999 (P=.45). Approximately 1.7 times more deaths in barrows than in gilts were recorded as death from any other cause.
Mortality by weeks from time of fill. The weekly number of deaths recorded for the three major causes of death (volvulus, dermatitis-nephropathy syndrome, and gastric ulcer) increased almost linearly from close to zero at fill to the highest value at 15 weeks.
Discussion
The incidence of HBS in these two large integrated production units was similar to those recently reported.2,3 While the overall incidence on both farms was relatively low, HBS was responsible for a fifth to a third of all deaths. In this study, the pattern of the ages of pigs recorded as dying from HBS is a reflection of the accuracy of diagnosis made by farm personnel or veterinarians. Site managers made the diagnosis of death due to HBS on Farm 1 and may have differed in the criteria they used to arrive at the diagnosis.
While farm personnel on Farm 1 made their diagnosis of HBS after a cursory or no necropsy, veterinarians on Farm 2 performed thorough necropsies on dead pigs. Finding a mesenteric twist in pigs with classical signs of HBS (sudden death of a previously healthy, well-grown pig and a pale carcass with a bloated abdomen containing a thin-walled small intestine filled with gas and either clotted or unclotted blood) provides evidence that a mechanical twisting of the intestine with circulatory impairment is the immediate cause of death. However, data from this study does not indicate a precipitating cause for the volvulus.
Gender differences in HBS deaths were compared only within site. For any cause of death, the data could be combined, since the recognition of a pig being dead would not differ by site. Also, although facilities were nearly identical in construction, feed was supplied from the same source, and pigs came from a common source, there may have been site differences. Because of potential site differences, comparisons were made within site whenever possible when factors were examined to determine their influence on HBS mortality. In these two data sets, as reported in other studies,10-12 the overall mortality of barrows was higher than that of gilts. It has been suggested that castration is responsible for the greater prevalence of disease in males due to stress and hormonal changes.10 Failure to find a difference in HBS incidence between genders on the two farms examined supports the findings of two studies in which gender was not a factor predisposing to HBS,13,14 and disagreeswith studies that reported a higher incidence in boars than in gilts or barrows15 or a higher incidence in females.7 Because diseases for which there are gender differences in prevalence tend to be infectious, the lack of gender difference for HBS supports a non-infectious etiology.10-12
Occurrence of a wide range of infectious diseases depends greatly on environmental and managerial factors. Farm conditions that allow one pathogen to cause clinical disease also allow other pathogens to produce disease. Lack of correlation of HBS with other (primarily infectious) causes of death suggests that HBS is not caused by a primary infectious agent. Microbiologic investigations have focused on clostridial organisms, Escherichia coli, and salmonellae, and while a specific infectious agent has not been associated with HBS, addition of antibacterials to the ration was successful in reducing the number of cases seen in three affected herds.2,3
In this study, HBS mortality was lower in pigs fed rations containing chlortetracycline, bacitracin, or bacitracin combined with an arsenical compound compared to pigs fed rations containing virginiamycin or no antibiotic. Antibiotics may inhibit overgrowth of normal gut microflora that proliferate under certain conditions to produceHBS. However, it is also possible that antibiotics influence occurrence of HBS by a physiologic mechanism that reducesthe risk of intestinal volvulus, for instance, slowing the passage of ingesta or changing the proportion of fluid in gut content. An alternate explanation for the perceived benefit of antibiotics is that the presence of antibiotics in the gut lumen after death may inhibit post-mortem proliferation of gas-forming bacteria. When necropsies are not performed, dead pigs that do not show the typical distended gut may not be reported as HBS, and as a result,an association might be made between the use of antibiotics and the number of HBS deaths recorded (B. Boswell, oral communication. August 2001).
On Farm 1, HBS occurred with greater frequency in the summer. Gastric ulceration is also seen more frequently in the summer.1,16 The higher incidence of HBS in the summer may suggest that alteration in feed intake is a contributing factor. Undertypical ad libitum feeding systems, pigs employ a feeding pattern of discrete meals with relatively long intermeal intervals. Most meals are taken during daylight hours.17 A close association of water-drinking with meal-eating has been found, where 75% of daily water intake occurred in the interval between 10 minutes before and 30 minutes after a meal, and 27% of total periprandial water was ingested just before the meal.17 As pigs become older and heavier, the number of daily feeding bouts decreases, and the average size and duration of each meal increases. In one study, the number of daily feeding bouts decreased from 13.5 to 6.6 as pigs grew from 10 to 120 kg.16 In another study, in pigs growing from 34 to 98 kg and housed four per pen, the number of daily feeding bouts decreased from 27 to 10 while the average meal size increased nearly threefold and the meal duration doubled.18 Lean pigs spend less time at the feeder and have a faster rate of feeding compared with obese pigs.19
Possibly, delivery of larger volumes of food to the intestine (with consumption of larger, less frequent meals) predisposes older pigs to HBS through purely mechanical mechanisms, ie, the weight of the ingesta is sufficient to rotate the gut. Ruckebusch and Bueno (1976)20 suggested that a combination of mechanical and physiologic mechanisms may be in play: "in pigs feeding disrupts the basic migrating myoelectric complex (MMC) pattern to varying extents, according to the volume of food ingested at one meal. The motor activity of the pig’s antrum and intestine after one large meal resembles that seen in carnivores, and the duration of this effect is approximately halved when two meals per day are given. When pigs are fed ad lib, the motility pattern resembles that seen in ruminants, where the MMC pattern persists regardless of feeding". Therefore, interruption of feeding, such as would occur if feeders became empty for a period of time, might be an inciting factor if gut stasis predisposesto HBS.
Fighting, playing, mounting, and other puberty-related behaviors might produce intestinal volvulus, especially if these activities occur when pigs are reducing feeding frequency and consequently increasing meal size. Modern confinement systems may allot only 0.7 m2 of pen space per pig, and aggressive behavior is more common among pigs allowed less than this.21
The longer carcass of modern swine compared to those raised 20 or more years ago might increase the possibility of volvulus. Smith and Lunney found a twofold difference in incidence of HBS between Large White and Landrace pigs.14
Because numerous reports associated whey feeding with outbreaks of HBS, the hypothesis was proposed that a hypersensitivity reaction mediated by heterologous milk proteins may induce HBS.1,13,22,23 Jones demonstrated antibodies to milk constituents in whey-fed pigs, but did not prove an association between presence of antibody and occurrence of HBS.13 Whey-fed pigs consume large volumes of liquid, which might predispose to volvulus.
In this study, a reduction in pig density was not associated with a change in HBS mortality. However, it cannot be concluded that pig density does not influence the occurrence of HBS, because reduction in pig density coincided with the stress of sorting and (or) removing pigs. These two factors might negate each other if one reduces and the other increases risk of HBS.
A complete explanation for the occurrence and pathogenesis of HBS is still lacking. However, findings of this study, ie, lack of gender differences, failure to parallel age patterns of mortality typical of primary infectious diseases, seasonal patterns, and observation of volvulus on necropsy, supporta non-infectious etiology. Investigation of feed or water intake patterns would be a logical area for subsequent investigation.While an infectious component has not been demonstrated, reduction of HBS mortality with in-feed administration of chlortetracycline, bacitracin, or bacitracinplus an arsenical compound supportsthe use of these products on farms where HBS occurs.
Identifiable volvulus of the intestine has been a variable finding in necropsies of pigs with HBS. If volvulus is always present in HBS, it suggests a specific physical etiology, with secondary overgrowth of normal or pathogenic microflora. Veterinarians specifically attempting to identify volvulus can improve their chances of success if they don a disposable sleeve, make a small incision in the abdominal wall, and palpate the mesenteric root before opening the abdomen and allowing viscera to spill out (H. Tilstra, oral communication. August 2001).
Implications
- HBS is responsible for a considerable portion of finishing death loss.
- Rates of death attributed to HBS are lower when bacitracin methylene disalicylate or chlortetracycline are administered in the feed than when feed contains virginiamycin or no antibiotic.
- To further investigate whether HBS and volvulus are two separate syndromes, veterinarians and farm personnel trained to perform necropsies should palpate the mesenteric root before opening the abdomen.
References – refereed
1. Veterinary Investigation Service. A survey of the incidence and causes of mortality in pigs. 1. Sow survey. Vet Rec. 1959;37:777-786.
3. Schultz RA, Daniels GN. Use of BMD to control hemorrhagic bowel syndrome in swine. Vet Med. 1984:79;253-256.
5. Smith WJ, Shanks PL. Intestinal haemorrhage syndrome. Vet Rec. 1971;89:55-56.
6. Rowland AC, Lawson GHK. Intestinal haemorrage syndrome in the pig. Vet Rec. 1973;93:402.
7. Häni H, Zimmermann W, Huber A, Schmidt J. Das "hämorrhagische Intestinalsyndrom" (HIS) des Schweines: Klinische, pathologisch-anatomische und ätiopathogenetische Aspekte. Schweiz Arch Tierheilk. 1993;135:117-124.
8. Kersting KW, Thompson JR. Diseases of the ruminant forestomach. In: Howard JL, Smith RA, eds. Current Veterinary Therapy. 4th ed. Philadelphia, Pennsylvania: WB Saunders Co; 1999:507-510.
9. Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology. Ames: Iowa State Univ Press; 1987.
10. Christensen G, Sørensen V, Mousing J. Diseases of the respiratory system, In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:913-940.
11. Kruijf JM, Welling AAWM. Het voorkommen van chrinische onstekingen bij gelten on borgen. [Occurrence of chronic inflammatory conditions in gilts and castrated male pigs]. Tijdschr Diergeneeskd. 1988;113:415-417.
12. Cowen P, Li SG, McGinn TD. Survey of trichinosis in breeding and cull swine using an enzyme-linked immunosorbent assay. Am J Vet Res. 1990;51:924-928.
13 Jones JET. An intestinal haemorrhage syndrome in pigs. Br Vet J. 1967;123:286-293.
16. Nienaber JA, Hahn GL, McDonald TP, Korthals RL. Feeding patterns and swine performance in hot environments. Trans Amer Soc Agric Eng. 1996;39:195-202.
17. Houpt TR. Controls of feeding in pigs. J Anim Sci. 1984;59:1345-1353.
18. Nienaber JA, McDonald TP, Hahn GL, Chen YR. Group feeding behavior of swine. Trans Amer Soc Agric Eng. 1991;34:289-294.
19. Wangsness PJ, Gobble JL, Sherritt GW. Feeding behavior of lean and obese pigs. Physiol Behav. 1980;24:407.
20. Ruckebusch Y, Bueno L. The effect of feeding on the motility of the stomach and small intestine in the pig. Br J Nutr. 1976;35:397-405.
21. Ewbank R, Bryant MJ. Aggressive behaviour amongst groups of domesticated pigs kept at various stocking rates. Anim Behav. 1972;20:21-28.
22. Todd JN, Jones TD, Morgan TLA, Francis SG, Hewitt SG. Intestinal haemorrhage and volvulus in whey fed pigs. Vet Rec. 1977;100:10-12.
23. Hobson, G. A haemorrhagic bowel syndrome in pigs. Vet Rec. 1971;89:27-28.
References – non refereed
2. Schultz RA, Daniels GN. Use of BMD to control hemorrhagic bowel syndrome in swine. Proc 8th IPVS Cong. 1984;363.
4. Lomax L. Hemorrhagic bowel syndrome. Proc AASP. Kansas City, Missouri. 1979;1-9.
14. Smith WJ, Lunney DC. Intestinal haemorrhage syndrome (var Jones) – epidemiologic studies in one herd over 6 years. Proc 4th IPVS Cong. 1976;N-4.
15. Allen MM, Saunders RW. The intestinal hemorrhage syndrome in centrally tested pigs in Great Britain. Proc 4th IPVS Cong. 1976;N-5.