Gregg D. 2002;1: Diagnostic notes: Update on classical swine fev
Diagnostic notes
Non refereed
Update on classical swine fever (hog cholera)
Douglas Gregg DVM, PhD
Foreign Animal Disease Diagnostic Laboratory, NVSL, APHIS, USDA.
Gregg D. Update on classical swine fever (hog cholera). J Swine Health Prod. 2002;10(1):33-37. Available as a PDF
Summary
Classical swine fever (CSF), commonly known in the United States as hog cholera, is a highly contagious viral disease of swine caused by a Pestivirus related to bovine virus diarrhea (BVD) and border disease virus (BDV) of sheep. Virulence varies from mild to severe. Most current outbreaks are associated with moderately virulent strains.1 The classic virulent disease is now rather uncommon. Classic highly virulent disease characteristically presents with high fever, extreme lethargy, hemorrhages in numerous organs, neurological signs, leukocytopenia, and high mortality. Moderately virulent disease causes high fever, mild lethargy, mild hemorrhages in lymphoid organs, transient leukocytopenia (or none), and low mortality.2 Recovered pigs may have a chronic disease with intermittent fever and poor reproductive performance. Pigs born of infected sows may be persistently infected and carry the virus for life, but with no immune response. The virus can also be transmitted in semen to sows. An outbreak of moderately virulent CSF may go undiagnosed for months due to its mild nature and few lesions.
Etiology
Classical swine fever virus (CSFV) is a lipid-enveloped virus in the family Flaviviridae, genus Pestivirus. The virus has one serotype, but many strains have antigenic similarity to BVD and BDV. Pestiviruses comprise a spectrum of strains that are difficult to distinguish serologically. Virus neutralization tests or monoclonal antibodies can differentiate CSFV from these related viruses, but multiple tests may be required. Both BVD and BDV have been isolated from swine in Europe. Bovine viral diarrhea occasionally causes a syndrome in pigs similar to that caused by CSF.3 Classical swine fever virus infects all swine including wild boar and peccaries, but is not known to infect cattle or sheep.
Geographic distribution
According to the 1996 Office International des Epizooties Manual, classical swine fever has a nearly worldwide distribution involving 44 swine-producing countries on all continents except North America and Australia.4 In Europe, CSF is endemic in wild boar in Italy, Germany, and parts of France and Switzerland. Countries of the European Economic Community no longer vaccinate for CSF but experience periodic outbreaks, often originating from wild boar. Reports from Asia are incomplete, but the disease is believed to be present in swine-producing areas and controlled by vaccination. Many countries of South America and Asia vaccinate for CSF. The most recent outbreaks in the Netherlands and England were both of Asian origin.
CSF: A disease on the move
Historically, CSF has moved with the transport of infected pork products. Since the earliest reports of hog cholera in Tennessee, this disease has been spread widely in cured hams and sausage. The recent outbreak in England in 2000 was suspected to have been introduced in ham that may have come from southeast Asia. Despite strict animal importation regulations, CSF-infected swine products are likely to be introduced into the United States by air, sea, or ground transportation in garbage or the baggage of travelers from endemic areas. Specialty ethnic pork products are highly valued and are regularly confiscated from international travelers at US airports. Smuggled pork products confiscated at the borders amount to hundreds of tons per year. The CSF virus can survive in fresh meat for 45 days at 4°C and for years at -20°C, and is quite resistant to inactivation by salting or curing. Virus survives for 85 days in cured smoked hams, and up to 180 days in salted and dried Sorrono hams, loins, or sausages.5 The virus is quite resistant to pH changes between 3 and 11, and can survive in the environment for months in contaminated soil of barnyards, particularly in temperate climates.6 Scraps of CSFV-infected fresh, cured, or insufficiently cooked meat fed to pigs can transmit the virus. Some international airlines and ships serve meals containing specialty pork products from their country of origin, and the protein-rich scraps may be fed to pigs at the destination. This common but risky practice of feeding to pigs garbage from air and sea ports should be prohibited, or at least carefully monitored cooking should be required.
Once introduced, CSFV usually spreads through livestock production systems either by direct movement of pigs or by fomites, farm workers, veterinarians, farm equipment, and vehicles.7 Infected pigs shed virus in all excretions including feces, urine, saliva, semen, and blood. Chronically or persistently infected sows may infect piglets in utero, and boars may shed virus in semen.1 Transmission is usually through ingestion, but also through skin abrasions, bites (fighting), and contaminated needles and instruments. Airborne, rodent, or insect transmission is possible over short distances but is not likely to cause distant spread. There is no evidence that CSFV replicates in insect vectors.
Introduction of CSF into a breeding herd is particularly devastating for several reasons. Once introduced, moderately virulent CSFV may cause only mild disease in mature sows and result in reproductive problems that may be overlooked or attributed to other causes. Sows may remain chronically infected for months and may farrow persistently infected litters.8,9 Some of these offspring are immunologically tolerant to CSFV and may carry high titers of virus for life. If CSFV enters a large agribusiness operation, persistently infected pigs would likely be moved hundreds and even thousands of miles between farrowing and finishing, spreading the disease widely before it is discovered.
The increasing popularity of wild boar meat has resulted in commercial trade in wild and feral swine carcasses. It is suspected that a number of European outbreaks have been caused by wild boar hunting, particularly in Germany, or importation of Asian wild boar meat.
CSF: Many faces of the same disease
The incubation period of CSF varies with the virulence of the virus, and ranges from 3 to 15 days. Under experimental conditions, 4 to 7 days is the average time from exposure to the onset of fever.
Clinical features of CSF vary widely, not only with virus virulence, but also with the age, breed, and condition of the host. Younger pigs are generally more susceptible than market weight hogs. Immunocom-promised pigs experience more severe disease. Pregnant sows are more susceptible than boars of the same age. Sows may have stillbirths or abortions or farrow persistently infected piglets. Pigs prone to porcine stress syndrome are paradoxically more resistant to CSFV.10
Since the late 1960’s, moderate-virulence and low-virulence strains have become more predominant in Europe and Central America. In Central America, this may be the result of intermittent vaccination used as a sole control measure to eliminate the more obvious virulent CSF.
The classic disease is severe, acute, and nearly always fatal. This form of CSF has received the most attention in veterinary textbooks but is now relatively uncommon. The initial signs are high fever and lethargy, trembling, and huddling or piling of pigs. They rapidly lose appetite and may vomit. Fever may reach 42.2°C for several days. Pigs have constipation initially and yellowish diarrhea in the last days. They continue to drink but only when aroused. Conjunctivitis may be marked and eyes may be heavily crusted with exudates (Figure 1). Pigs are reluctant to rise, stand with arched backs, and move with a swaying or staggering gait. Terminally, there may be purple discoloration of the skin of the abdomen, thighs, and ears. Convulsions may be seen at any time after onset of fever, and are often initiated by handling.
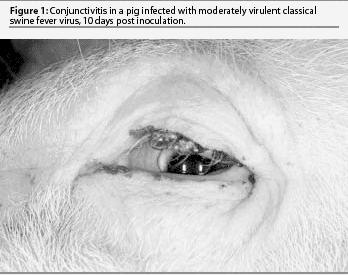
The initial clinical signs of moderately virulent CSF are similar but milder than those of highly virulent CSF. There is a longer incubation period (6 to 7 days) followed by a slight fever and mild lethargy. Fever rises slowly for several days and usually peaks at 40.5 to 41.1°C. Pigs prefer to huddle together but will rise, drink, and eat when disturbed. Appetite is reduced. Pigs appear weak upon rising, but there is little staggering and no convulsions. There may be mild constipation. Fever persists for 2 to 3 weeks with only trembling noted clinically. A few pigs develop moderate diarrhea. Remarkably, most pigs do not die, and the fever decreases or fluctuates through the fourth week. There may be conjunctivitis and poor weight gain, but pigs are typically not gaunt. There are few or no hemorrhages or areas of skin discoloration. A few pigs may die suddenly with pneumonia due to enteric bacteremia. Recovered pigs may appear normal and gain weight, but at a slower rate. Some recovered pigs are chronically infected for months, and following stress, have a sudden exacerbation of high fever for 2 to 3 days and die.
There are even less virulent strains that only cause a mild fever of 40 to 40.5°C for a week or two with no other signs. These pigs usually recover but may become chronic carriers.
The Trojan horse: Congenital persistent infection
Congenital infections of fetuses are common in sows with CSF. There may be a variety of sequelae, including abortion, stillbirth, mummification, or birth of weak and dying piglets. Cerebellar hypoplasia or hydrocephalus are rare congenital malformations.
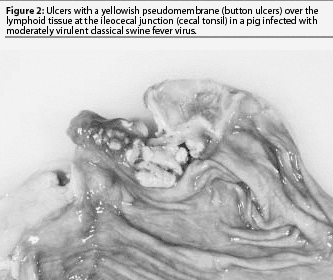
Some piglets exposed to CSFV in the first trimester may be born healthy, but because they were exposed before the immune system developed, become persistently infected with CSFV.8,9 These pigs are likely to have multiple secondary bacterial or viral infections and will not be suspected of having CSF. They may live for weeks or up to a year. Most will be chronic poor doers and gain weight slowly. Clinical signs attributed to CSF include depression, conjunctivitis, dermatitis, diarrhea, ataxia, and death. Secondary infections with other viruses or bacteria usually dominate the clinical picture. A common cause of death is severe bacteremia and pneumonia resulting from bacterial invasion through ulcers at the ilio-cecal junction (button ulcers) (Figure 2). Persistent CSFV infections are difficult to diagnose and may spread to other herds by animal movement or artificial insemination.11 Poor reproductive performance may draw attention to such herds.
Pathology
Virulence of CSFV strains is closely linked to their tissue tropism. Virulent CSFV is pantropic, infecting many tissues, particularly endothelium, epithelium, lymphoid organs, and endocrine tissue. Endothelial infection results in petechial hemorrhages in the renal cortex, intestinal serosa, larynx, lungs, urinary bladder, and skin. Epithelial infections may occur throughout the digestive tract. Tonsillar epithelium is infected and there may be crypt necrosis and abscesses. Changes in the small and large intestine may include catarrhal exudate, hemorrhage, erosion, and ulceration. Diarrhea results from epithelial erosion and atrophy. Ulceration is more common in long-standing infections. Lymphoid lesions are the most prominent and consistent, resulting in lymph node swelling, necrosis, and variable hemorrhage in the submandibular, gastrohepatic, renal, and mesenteric lymph nodes. The spleen may have raised, dark, firm infarcts along its margin. Though splenic infarcts are uncommon and are most often seen in the virulent form of the disease, they should alert you to think of CSF.
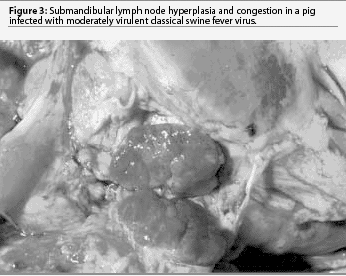
Today, moderately virulent CSFV causes much milder gross lesions that reflect a limited tissue tropism, primarily epithelial and lymphoid infection. Without complications, most pigs recover. Congestion and sometimes hemorrhages may be seen in the gastro-hepatic and submandibular lymph nodes (Figure 3). A few pigs may die after several weeks as a result of enteritis, with erosions or ulcers developing in the cecal tonsil or spiral colon (button ulcers). After several weeks of disease, chondrodysplasia may cause widening of the costochondral junction (Figure 4). In some cases, fulminating acute enteric bacteremia with diffuse pneumonia causes death. There may also be focal liver necrosis and hemorrhages in various organs, including mesenteric lymph nodes. Only careful examination reveals cecal or colonic ulcers as the source of the bacteremia. It is unlikely that CSF would be considered as a differential in these mild cases.

Low-virulence CSFV causes no gross lesions in young piglets. Sows may experience reproductive failure manifested as abortions, stillbirths, or mummification. Congenital anomalies are rare.
Diagnosis
The palatine tonsil is the site of early infection and the tissue most consistently positive for virus. It is the preferred tissue for CSFV immunostaining and virus isolation. Both the direct fluorescent antibody (DFA) test and immunohistochemistry demonstrate CSFV antigen in the tonsillar crypt, surface epithelium, and germinal centers. Other important tissues are the submandibular, gastrohepatic, and mesenteric lymph nodes, thymus, spleen, kidney, lower ileum, cecal tonsil, and brain. Histopathology is most useful as an adjunct for CSF diagnosis if combined with immunohistochemical staining. With relatively virulent CSF, pigs that die often have histopathological lesions of marked lymphoid necrosis and atrophy of germinal centers in lymph nodes and spleen. Hemorrhage may be present in many organs, but is a nonspecific finding. Antigen may be demonstrated in vascular endothelium in pigs with virulent CSF. There is often non-specific mild encephalitis with perivascular cuffing of lymphocytes, and microgliosis.
Differential diagnosis
The list of differential diagnoses for CSF must include any highly contagious febrile disease of pigs. The most likely differential in the United States is salmonellosis, but the list also includes other important diseases like virulent porcine respiratory and reproductive syndrome and swine erysipelas. Fibrinohemorrhagic typhlocolitis may be present in CSF, salmonellosis, swine dysentery, or Escherichia coli infection in weanling pigs. With chronic CSF, bacteremia or acute bacterial pneumonia is a common complication. Dermatitis-nephropathy syndrome causes petechial hemorrhages in the renal cortex, hemorrhagic skin lesions, and possibly splenic infarcts resembling those of virulent CSF. Salt poisoning-water deprivation may cause acute death and encephalitic signs that mimic CSF. Other important viral differentials include African swine fever and pseu-dorabies.
Sample collection and submission
Field diagnosis
Any investigation of a septicemic disease of pigs causing prolonged high fever should include CSF in the differential. A thorough history, searching for evidence of contact with raw garbage, recent addition of pigs of unknown origin, or contact with people who have traveled internationally, is recommended. Examine and send for laboratory diagnosis samples from sick, dying, and dead pigs. Blood counts may be useful, but are sometimes abnormal only immediately after the initial rise in body temperature. An initial lymphopenia may persist for a few days, but the lymphocyte differential may return to normal or be masked by neutrophilia in a total leukocyte count. Because of the frequency of secondary diseases in a CSF outbreak, a presumptive diagnosis should not be based on only one necropsy. A definitive field diagnosis of CSF is not possible with the current strains of virus. Since there are few pathognomonic lesions of CSF (and sometimes few lesions at all), an investigation of any disease of high morbidity with persistent fever in pigs should include a laboratory examination of tissues for CSF.
Laboratory diagnosis
The diagnosis of CSF requires laboratory confirmation by a reference laboratory. Heparinized blood should be collected for virus isolation.1 Samples should be collected from several pigs in various stages of disease, as well as from all available dead pigs. All samples should be individually collected in plastic bags and shipped on ice packs in leakproof containers. Avoid freezing as it will create tissue section artifacts. Submit a full set of tissues, including the entire brain, in 10% buffered formalin. Fresh tonsils will be examined by DFA. The DFA test uses a polyclonal antiserum, thus BVD or BDV viral antigens in swine tonsils may cross-react in a small percentage of cases.7 Positives or suspects by the DFA test must be confirmed by additional tests.12,13 Monoclonal antibody staining, virus isolation, and differentiation by serum neutralization can distinguish these related pestiviruses.3 Monoclonal antibody immunostaining takes a few hours, but virus isolation and neutralization take a week or more. The polymerase chain reaction (PCR) test is a useful and relatively rapid method for detection and differentiation of pestiviruses.8 In countries that have been free of CSF, the initial case should be confirmed by pig inoculation in a high-containment laboratory with re-isolation of virus.
Moderate-virulence and low-virulence strains of CSFV cause such a mild disease that outbreaks may not be suspected or investigated, particularly if death losses are low. Continuous, random serological surveillance programs are important, and may be the only means of detecting occurrence of these mild forms of CSF. Feral and wild pigs often range widely and come into contact with domestic swine. They should be included in this surveillance. There are a number of serological tests used for this purpose, including enzyme-linked immunosorbent assays (ELISAs), complex trapping blocking ELISA, and indirect immunoperoxidase tests.14 It is important to note that antibody is detectable for only 2 to 3 weeks after onset of disease. Therefore, serology may not be useful in detecting disease during most of the clinical course.4
Vaccination complicates the interpretation of all diagnostic tests. Modified live virus vaccines replicate in the tonsil and other lymphoid tissues, and antigen is detectable for up to 10 days after vaccination.4 The C-strain vaccine can be distinguished from field viruses by direct immunoperoxidase staining of tissue sections, but this requires an additional test using monoclonal antibodies. Animals vaccinated with conventional vacccines cannot be serologically distinguished from pigs recovered from CSF.
Zoonotic potential: Public health implications
Human beings are not susceptible to infection with CSFV.
Control, eradication, and vaccination
Control and eradication are vital for economic reasons. Vaccination is employed in many countries to control outbreaks and has been used effectively in systematic and consistent programs to reduce disease outbreaks to a point where eradication measures are feasible.11 Currently, nearly all vaccines on the market are modified live virus vaccines rendered non pathogenic for pigs over 2 weeks of age and are safe to use in pregnant sows. These include the lapinized Chinese (C) strain, Japanese guinea pig cell-culture-adapted strain, and French Triverval strain.7 Subunit vaccines based on the sequence of the E2 region are currently available and have proven effective in experimental trials.11 At the time of this writing, they have not been used in the field. It is possible to serologically distinguish pigs that have been vaccinated with a subunit vaccine from pigs that have recovered from a field strain of CSFV.
Vaccination allowed the United States and most countries of the European Union to successfully reduce CSF to the point that sanitary measures could eradicate the disease. This requires a strong veterinary infrastructure, diagnostic laboratory system, and strong support from the government and swine industry. Indemnities must be paid promptly and be generous enough to encourage reporting of disease. To prevent new outbreaks, strict importation restrictions must be applied to pigs and pork products originating from countries where CSF is present. Garbage feeding must be stopped or carefully regulated to ensure proper cooking. Care must be taken to destroy all port garbage.
Laboratories must be well staffed and funded to keep pace with an eradication program. Rapid completion of serological surveys and case investigations reduce the need for field personnel to maintain lengthy quarantines. Once eradication is near completion, serological surveillance must be intensified to identify carrier sows in breeding herds. Surveillance must then be continued indefinitely in high risk areas near borders, ports, and garbage-feeding operations to insure there is no re-introduction.
References — refereed
2. Crauwels APP, Nielen M, Stegeman JA, Elbers ARW, Dijkhuizen AA, Tielen MJM. The effectiveness of routine serological surveillance: case study of the 1997 epidemic of classical swine fever in the Netherlands. Rev sci tech off int epizoot. 1999;18:627-637.
3. Wensvoort G, Terpstra C, Kluijver EP, Kragten C, Warnaar JC. Antigenic differentiation of pestivirus strains with monoclonal antibodies against hog cholera virus. Vet Microbiol. 1989;21:9-20.
4. Office International des Epizooties. Manual of Standards for Diagnostic Tests and Vaccines. Lists A and B diseases of mammals, birds, and bees. 3rd ed. 1996:145-154.
5. Mebus CA, Arias M, Pineda JM, Tapiador J, Housse C, Sanchez-Vizcaino JM. Survival of several porcine viruses in Spanish dry-cured meat products. Food Chem. 1997;59:555-559.
6. Horzinek MC. Non-Arthropod Borne Togaviruses. New York: Academic Press; 1981.
7. Biront P, Leunen J. Vaccines. In Liess B, ed. Classical Swine Fever and Related Viral Infections. Boston, Massachusetts: Nijhoff; 1988:181-197.
8. Dahle J, Liess B. A review on classical swine fever infections in pigs: epizootiology, clinical disease and pathology. Comp Immun Microbiol Infect Dis. 1992;15:3:203-211.
9. Van Oirschot JT. Experimental production of congenital persistent swine fever infections I. Clinical, pathological and virological observations. Vet Microbiol. 1979;4:117-132.
10. Depner KR, Hinrichs U, Bickhardt K, Greiser-Wilke I, Pohlenz J, Moenning V, Liess B. Influence of breed-related factors on the course of classical swine fever virus infection. Vet Rec. 1997;140:506-507.
11. Bouma A, De Smit A J, De Jong MC, De Kluijver EP, Moormann RJ. Determination of the onset of the herd-immunity induced by the E2 subunit vaccine against classical swine fever virus. Vaccine. 2000;18:1374-1381.
12. De Smit AJ, Bouma A, Terpstra C, van Oirschot JT. Transmission of classical swine fever virus by artificial insemination. Vet Microbiol. 1999;67:239-249.
13. Ressang AA, den Boer JL. A comparison between the cell culture, frozen tissue section, impression and mucosal smear techniques for fluorescent antibody in the diagnosis of hog cholera. Neth J Vet Sci. 1968;1:72.
14. Elbers ARW, Stegeman JA, Moser H, Ekker HM, Smak JA, de Leeuw PW. The classical swine fever epidemic 1997-1998 in the Netherlands: Descriptive epidemiology. Prev Vet Med. 1999;42:157-184.
References — non refereed
1. De Smit AJ, Terpstra C, Wensvoort G. Comparison of virus isolation methods from whole blood or blood components for early diagnosis of CSF. Report on Meeting of the EU National Swine Fever Laboratories, Brussels, Nov 24-25, 1994. 1994:22-24.