Harms PA. 2002;1:27-30 Three cases of porcine respiratory diseas
Case study
Peer reviewed
Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection
Perry A. Harms, DVM, MS; Patrick G. Halbur, DVM, PhD; Steven D. Sorden, DVM, PhD, Dipl ACVP
PAH, PGH, SDS: Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 50011.
Harms PA, Halbur PG, Sorden SD. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J Swine Health Prod. 2002;10(1):27-30. Available as a PDF
Summary
Pneumonia associated with porcine circovirus type 2 (PCV2) is being diagnosed in an increasing number of respiratory disease cases in growing swine. Multiple viral infections are commonly diagnosed in cases of porcine respiratory disease complex (PRDC) at the Iowa State University Veterinary Diagnostic Laboratory. In the year 2000, porcine reproductive and respiratory syndrome virus (PRRSV) was present in 42% of these cases, PCV2 was present in 22%, swine influenza virus (SIV) was present in 19%, and Mycoplasma hyopneumoniae was present in 22%. In this report, three clinical cases are described in which there were concurrent infections with PCV2 and PRRSV, PCV2 and SIV, and PCV2 and M hyopneumoniae. Prolonged and unusually severe clinical disease, unique histologic lesions of lymphoid depletion and airway fibrosis, and abundant PCV2 antigen within these lesions suggest that PCV2 is playing a pathogenic role in PRDC.
Keywords: swine, porcine circovirus
type 2, porcine reproductive and respiratory syndrome virus, swine
influenza virus, porcine respiratory disease complex
swine, porcine circovirus
type 2, porcine reproductive and respiratory syndrome virus, swine
influenza virus, porcine respiratory disease complex
Received: May 14, 2001
Accepted: August 7, 2001
Respiratory disease is a major health
problem in growing swine in the United States. Cases of respiratory
disease involving multiple pathogens are often referred to as
porcine respiratory disease complex (PRDC). Porcine reproductive
and respiratory syndrome virus (PRRSV) and swine influenza virus
(SIV) are the common viral agents associated with PRDC. Mycoplasma
hyopneumoniae, Pasteurella multocida, Streptocccus
suis, and other bacterial agents may also be associated. Porcine
circovirus type 2 may be identified in cases of pneumonia. However,
it has most often been associated with postweaning multisystemic
wasting syndrome (PMWS),1-4 which is characterized
by wasting, dyspnea, and occasionally jaundice or pallor. This
syndrome commonly occurs in pigs between 8 and 16 weeks of age.
The defining histologic features of PMWS include depletion of
lymphocytes in lymphoid tissues, occasional intracytoplasmic inclusion
bodies, and lympho-
histiocytic to granulomatous inflammation in multiple organs,
such as intestine, kidney, lymphoid tissue, liver, and lung. Interstitial
pneumonia is evident histologically in many cases.3, 5-7
Several reports1-4, 8 describe cases of PMWS associated
with PCV2; however, the literature lacks clinical reports describing
the involvement of PCV2 in PRDC. The following case reports exemplify
the association of PCV2 with PRDC in the accessions of a US diagnostic
laboratory with a heavy swine case load.
Case reports
In the year 2000, swine pneumonia was diagnosed in 3163 cases presented to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL)(Table 1). Diagnosis was based on gross and microscopic lesions and demonstration of an agent by isolation, antigen detection, or identifica-tion of nucleic acid by polymerase chain reaction (PCR) or in situ hybridization in the tissues. The most common etiologic diagnosis was PRRSV, in 42% of the swine pneumonia cases. Porcine circovirus type 2 was diagnosed in 22% of cases, SIV in 17%, and M hyopneumoniae in 14%.

Porcine circovirus type 2 was commonly found in combination with other pathogens. Of the 694 cases that were diagnosed with PCV2-associated pneumonia, both PCV2 and PRRSV were demonstrated in 388 (56%), which is greater than the 42% prevalence of PRRSV in the total pool of pneumonia cases, suggesting an interaction between these two viruses. Concurrent infection with PCV2 and M hyopneumoniae was confirmed in 134 of the 694 cases (19%), and concurrent infection with PCV2 and SIV was demonstrated in 83 cases (12%).
Case One: Concurrent infection with PCV2 and PRRSV
A group of 2000 pigs averaging 34 kg experienced respiratory disease 2 to 3 weeks after placement in a finishing barn. The primary clinical signs, observed in 10 to 15% of the pigs, were inactivity, anorexia, and dyspnea. Though anorexia was observed in some pigs, wasting was not a presenting sign. At necropsy, lungs of affected pigs failed to collapse and were diffusely mottled, red to purple, and rubbery. No other gross lesions were present. Fresh and formalin-fixed tissues (lung, tonsil, spleen, and ileum) from two pigs representative of the group (healthy except for respiratory signs) were submitted to the ISU-VDL early in the course of the outbreak.
Histopathology revealed severe, diffuse, lymphohistiocytic interstitial pneumonia with marked type 2 pneumocyte hyperplasia and hypertrophy, necrotic cellular debris within alveolar spaces, moderate multifocal suppurative bronchopneumonia, and mild peribronchiolar fibrosis. Mild lymphocyte depletion was observed in Peyer’s patches, but not in tonsil or spleen. Numerous cells positive for PCV2 antigen were detected in the alveolar septa and around bronchioles by immunohistochemistry (IHC) using antibodies derived against PCV2, and alveolar macrophages were positive for PRRSV antigen by IHC.9 Small numbers of non-pathogenic bacteria were isolated from the lungs, spleen, and small intestine. Mycoplasma hyopneumoniae was not detected by direct immuno-fluoresence assay (IFA), nor was SIV detected by IHC in the lung tissues. On the basis of these findings, the diagnosis was severe interstitial pneumonia due to concurrent PCV2 and PRRSV infections.
Although the pigs were treated with antibiotics in the water and feed, new pigs continued to become affected for a 3- to 4-week period, with a total of 5% of the pigs removed and placed in hospital pens. Many pigs continued to lose body condition despite individual treatments and were euthanized for humane reasons. Total mortality for the group was 9%, with an additional 8% marketed as cull pigs at a significantly reduced value.
Case Two: Concurrent infection with PCV2 and SIV
This farm received feeder pigs weighing 23 kg from a single source every 3 weeks. One group of 700 pigs, housed in outside lots and weighing 54 to 64 kg, experienced an acute onset of respiratory disease with clinical signs (coughing, dyspnea, and lethargy) in 40% of the pigs. Field necropsies revealed tan-to-red, mottled lungs that failed to collapse, and cranioventral consolidation affecting 20 to 30% of the lung. No other gross lesions were present. On day 3 of the outbreak, necropsies were performed on two pigs selected to represent the group, and fresh and formalin-fixed lung tissues were submitted to the ISU-VDL for further investigation.
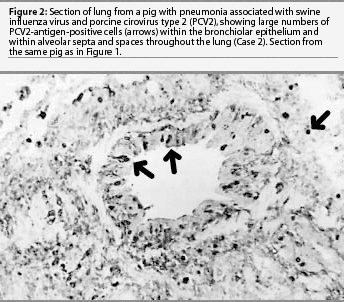
Lung was the only formalin-fixed tissue examined. Histopathology revealed multifocal bronchial and bronchiolar epithelial necrosis, squamous metaplasia, and hyperplasia. Moderate to marked multifocal peribronchial and peribronchiolar fibrosis and macrophages were present and often extended into the airway lamina propria. In addition, there was multifocal suppurative bronchopneumonia. Swine influenza virus antigen was identified by IHC in the nuclei of numerous bronchial and bronchiolar epithelial cells and occasional cells within the lung parenchyma (Figure 1). Immunohistochemistry also demonstrated large amounts of PCV2 antigen both within bronchial and bronchiolar epithelial cells and in mononuclear cells surrounding the airways (Figure 2). Swine influenza virus H1N1 and Pasteurella multocida type A were isolated from the lung tissue. Immunohistochemistry did not detect PRRSV antigen, and direct IFA on frozen lung sections did not detect M hyopneumoniae antigen. The diagnosis was bronchiolitis and bronchopneumonia due to SIV, PCV2, and P multocida infections.

The outbreak lasted for approximately 2 weeks, with the group experiencing mortality near 10% despite aggressive antibiotic therapy. Five percent of the pigs failed to reach market weight and were sold at a reduced value.
Case Three: Concurrent infection with PCV2 and M hyopneumoniae
A group of 300 pigs weighing 77 to 82 kg experienced 10% morbidity and 2% mortality, with clinical signs of gauntness and dyspnea, over a 2- to 3-week period. Two pigs were selected to represent the more recently affected pigs in the group. Nec-ropsy revealed dark purple cranioventral consolidation of 20 to 30% of the lungs, and no other lesions.
Histopathology revealed fibrinosuppurative pleuritis, multiple neutrophil-rich thrombi, mild to moderate peribronchiolar lymphoid hyperplasia, and mild fibrosis of the peribronchiolar alveolar septa. Lymphoid tissues were not examined histologically. Immunohistochemistry on formalin-fixed lung tissue demonstrated large amounts of PCV2 antigen within the alveolar septa and macrophages surrounding bronchioles. Mycoplasma hyopneumoniae antigen was detected by direct IFA on frozen lung tissue sections. Large numbers of P multocida type A were isolated from the lungs. Neither PRRSV nor SIV was detected by IHC or virus isolation. The diagnosis was PCV2-associated bronchointerstitial pneumonia with mycoplasmal pneumonia and pneumonic pasteurellosis. The pleuritis and pulmonary arterial thrombosis suggested a concurrent or earlier bacterial septicemia.
This group of pigs continued to cough despite treatment with chlortetracycline (22 mg per kg) in the feed for an extended period. The group took longer to close out than normal. Mortality was less than 5%, but many of the deaths occurred in older pigs at heavier weights, when investments in feed, time, and facilities are greater.
Discussion
These cases represent PRDC in pigs infected concurrently with a recognized swine respiratory pathogen and PCV2. Case One (concurrent infection with PCV2 and PRRSV) is representative both clinically and diagnostically of a large number of cases of swine respiratory disease submitted to the ISU-VDL. These pigs were affected in the early- to mid-finishing period, experiencing severe respiratory disease with a prolonged recovery period, and a large percentage of the affected pigs died or did not recover well (17% deaths and culls in this case). Interstitial pneumonia with peribronchiolar fibrosis and identi-fication of both PCV2 and PRRSV in the tissues are common findings in similar cases.
Case Two (concurrent infection with PCV2 and SIV) represents acute outbreaks of respiratory disease. This case differs from Case One in the sudden onset of disease and is also in contrast to the chronic nature of a "wasting" disease as previously described for PMWS. Case Two had an acute clinical onset typical of a swine influenza outbreak, but microscopic lesions, such as peribronchiolar fibrosis and infiltrates of macrophages, were more chronic, recovery was prolonged, and mortality was much higher than is commonly associated with swine influenza.10
Case Three (concurrent infection with PCV2 and M hyopneumoniae) is included because the clinical features are consistent with those commonly seen in PRDC, but no evidence of PRRSV or SIV, the most frequently identified viruses, was demonstrated. Failure to detect PRRSV or SIV by IHC or virus isolation does not preclude the presence of either virus. However, the absence of characteristic lesions suggests that neither virus was contributing to the disease in these pigs.
Lymphoid depletion was mild and inconsistent in Case One, and in Cases Two and Three, lymphoid tissues were not examined, so the full extent of PCV2 infection is not known. Some pigs on which necropsies were performed may have had undetected lymphoid lesions characteristic of PMWS and might have progressed to develop PMWS. The variability in growth rate subsequent to the respiratory disease in the pigs in these cases may have been due to chronic effects of respiratory disease or development of PMWS in some animals. Regardless of their PMWS status, these cases are significant because of the contribution of PCV2 to the respiratory disease observed. Many PRDC outbreaks may be complicated by PMWS. These two diseases occur in pigs of similar ages; therefore, consideration of PCV2 as a possible pathogen in PRDC should be independent of the diagnosis of PMWS.
Infection with PCV2 was clearly demonstrated in these cases, but because most pigs seroconvert to PCV2 prior to slaughter,11,12 it might be argued that PCV2 infection was incidental. Disease in these pigs occurred during the early- to mid-finishing period, which coincides with the time when seroconversion to PCV2 commonly occurs.12 However, we believe that PCV2 played a significant role in the disease process in these herds for three reasons. First, clinical disease was longer, more severe, and resulted in greater numbers of low-weight pigs than would be expected with PRRSV or SIV alone. Second, fibrous tissue proliferation around airways has been associated with PCV2 infection,13 but has not been induced by experimental PRRSV or SIV inoculation. Third, the unique clinical signs and microscopic lesions observed were associated with numerous intrale-sional PCV2-antigen-positive cells.
In the year 2000, IHC for PCV2 was performed on 2030 swine cases submitted to the ISU-VDL at the request of the submitting veterinarians or due to suspicious clinical signs or lesions, but was positive in only 694 cases (34%). The pathogenesis of PCV2-associated disease is complex, and the role of PCV2 as a primary pathogen in PMWS or as a secondary respiratory pathogen is unclear. The classification of pathogens as primary or secondary often oversimplifies the interactions that occur in PRDC. Studies using a M hyopneumoniae and PRRSV coinfection model show that these pathogens can act synergistically to produce more severe respiratory disease than either pathogen alone.14 There is experimental evidence that synergism occurs between PCV2 and parvovirus15 and between PCV2 and PRRSV,16, 17 producing lesions and clinical signs of PMWS in both models as well as a respiratory component in the concurrent-PRRSV-infection model. While the primary or secondary role of PCV2 in PMWS or PRDC continues to be debated, the demonstration of unique lesions in the lungs associated with large amounts of viral antigen suggest that PCV2 is an important pathogen in some cases of PRDC.
Although we do not have a clear means to solve the problem, a diagnosis of PMWS or PCV2-associated disease is important for several reasons. Good production practices such as attention to pig flow, environment, pig density, and nutrition play an important role in preventing many diseases and are also recommended to moderate the severity of disease associated with PCV2.8 Secondly, identifying the involvement of PCV2 in an outbreak of PRDC enables the veterinarian to develop a more accurate prognosis for the group of pigs. The course of the disease may be more severe, prolonged, and refractory to therapy than PRDC uncomplicated by concurrent PCV2 infection. Understanding the potential outcome, the veterinarian and producer can make informed decisions regarding the choice and effectiveness of antimicrobial therapy in controlling secondary bacterial infections in PRDC. Thirdly, identifying PCV2-associated disease in a group of pigs allows swine veterinarians to monitor disease outcomes as they make management changes, develop intervention strategies, or make disinfectant choices18 for control of the PCV2-associated disease. Systematic evaluation of intervention strategies by practitioners will help us all in developing effective control strategies.
Implications
- Disease associated with PCV2 infection is most often found in conjunction with one or more other pathogens.
- Infection with PCV2 is commonly demonstrated in outbreaks of PRDC and may be contributing to the severity of the lesions and the severity and duration of clinical disease in these cases.
- Lesions associated with PCV2 infections can be found in acute respiratory disease and are not limited to a wasting syndrome.
Acknowledgements
We gratefully acknowledge Dr Jeffery Blythe, Dr Douglas Quam, and Dr Thomas Pollack for the original submissions and follow-up on these cases.
References — refereed
2. Meehan BM, McNeilly F, Todd D, Kennedy S, Jewhurst VA, Ellis JA, Hassard LE, Clark EG, Haines DM, Allan GM. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J Gen Virol. 1998;79:2171-2179.
3. Morozov I, Sirinarumitr T, Sorden SD, Halbur PG, Morgan MK, Yoon KJ, Paul PS. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J Clin Microbiol. 1998;36:2535-2541.
4. Nayar GP, Hamel A, Lin L. Detection and characterization of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. Can Vet J. 1997;38:385-386.
5. Rosell C, Segales J, Plana-Duran J, Balasch M, Rodriguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999;120:59-78.
6. Allan GM, McNeilly F, Kennedy S, Daft B, Clarke EG, Ellis JA, Haines DM, Meehan BM, Adair BM. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest. 1998;10:3-10.
7. Kiupel M, Stevenson GW, Mittal SK, Clark EG, Haines DM. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet Pathol. 1998;35:303-307.
9. Sorden SD, Harms PA, Nawagitgul P, Cavanaugh D, Paul PS. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest. 1999;11:528-530.
10. Easterday BC, Reeth KV. Swine influenza. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, IA: Iowa State University Press;1999:277-290.
11. Tischer I, Bode L, Peters D, Pociuli S, Germann B. Distribution of antibodies to porcine circovirus in swine populations of different breeding farms. Arch Virol. 1995;140:737-743.
12. Walker IW, Konoby CA, Jewhurst VA, McNair I, McNeilly F, Meehan BM, Cottrell TS, Ellis JA, Allan GM. Development and application of a competitive enzyme-linked immunosorbent assay for the detection of serum antibodies to porcine circovirus type 2. J Vet Diagn Invest. 2000;12:400-405.
14. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999;37:620-627.
15. Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan GM. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J Comp Pathol. 2000;122:9-24.
16. Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000;145:2421-2429.
17. Harms PA, Sorden SD, Halbur PG, Bolin S, Lager K, Morosov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and PRRSV. Vet Pathol. 2001;38:528-539.
References — non refereed
1. Harding JCS, Clark EG. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 1997;5:201-203.
13. Sorden SD, Thacker BJ, Harms PA, Siranarumitr T, Morosov I, Paul PS. Retrospective study of porcine circovirus infection in porcine tissues submitted to the Iowa State University Veterinary Diagnostic Laboratory using in situ hybridization. Proc Am Assoc Vet Lab Diagn. San Diego, CA. October 1999;55.