Practice tip |
Non refereed |
Serologic profiling and vaccination timing for Lawsonia intracellularis
D. Walter, DVM; C. Gebhart, PhD; J. Kroll, BS; J.T. Holck, DVM, MS, MBA; W. Chittick, BS
DW, JK, JTH, WC: Boehringer Ingelheim Vetmedica, Inc, Ames, Iowa. CG: Department of Pathobiology, College of Veterinary Medicine, University of Minnesota, St Paul, Minnesota. Corresponding author: Dr Don Walter, 1051 Barrington Road, Iowa City, IA 52245; Tel: 319-354-5902; Fax: 319-354-5902; Mobile: 319-321-2122; E-mail: dwalter@bi-vetmedica.com.
Cite as: Walter D, Gebhart C, Kroll J, et al. Serologic profiling and vaccination timing for Lawsonia intracellularis. J Swine Health Prod. 2004;12(6):310-313.
Also available as a PDF.
The purpose of this practice tip is to provide guidance to practitioners regarding integrated use of currently available Lawsonia intracellularis serology and immunization tools. Enterisol Ileitis (Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri), an avirulent live L intracellularis vaccine, is available for oral administration to pigs 3 weeks of age or older by drench or via the drinking water.1
Vaccination timing
Ideally, vaccines are administered when the proportion of animals with residual maternal immunity approaches 0% while the proportion of animals already naturally infected is still near 0%. Lawsonia intracellularis vaccine should be administered at least 3 to 4 weeks prior to the anticipated onset of infection with L intracellularis ("onset of immunity" lag period), so that protective immunity is present when pigs are first exposed to infection. Seroconversion usually occurs at least 2 to 4 weeks postinfection in artificial infection studies ("infection indicator" lag period). Using seroconversion as a means of estimating when vaccination should be scheduled requires that both lag periods be added together in order for vaccination to be scheduled sufficiently ahead of the infection indicator (seroconversion). Whenever possible and for best results, vaccination should be scheduled 8 weeks or more before the first pigs in the group are expected to seroconvert (Figure 1).
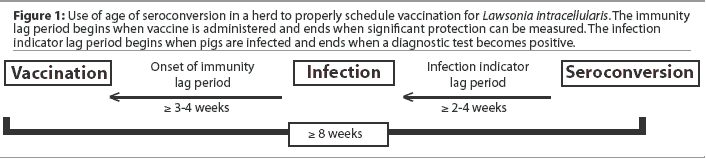
Additional vaccination timing considerations
Unpublished data (Boehringer Ingelheim Vetmedica, Inc, Research and Development Department) suggests that in pure culture challenge studies, immunity persists for at least 22 weeks following vaccination with Enterisol Ileitis. Therefore, vaccination of nursery pigs is likely to provide protection until pigs reach weights typical of North American pigs at slaughter. Field observations support this finding.2 The need to revaccinate breeding animals has not been determined, but initial field observations from the United States and Canada suggest that duration of immunity might be at least a year and that booster vaccination may not always be necessary.3
Antibiotics must be avoided for several days before and after vaccination, for a total nonmedicated period of at least 1 week, with vaccination on Day 4. The impact of other health challenges and their timing must also be taken into account. For example, if weaning precipitates streptococcal or other bacterial infections that require antibiotic medication during the immediate postweaning period, vaccination with L intracellularis vaccine may be delayed, provided that the selected antibiotic is also effective against L intracellularis. Unhealthy animals may not respond normally to any vaccination. It is important to note that the recommended 1-week non-medicated period is a minimum, and a longer antibiotic-free period, particularly postvaccination, may be advantageous in some cases.
It is best to avoid vaccinating pigs via the drinking water earlier than 4 days postweaning. Pigs may require several days to learn how to properly operate drinkers and thereby receive a full dose of vaccine. It may also take a day or two for the last milk (ie, residual lactogenic immunity) to transit the pig's gastrointestinal tract. However, maternal immunity to L intracellularis may persist for several weeks postweaning.4 Even though active immunization of weaned piglets against L intracellularis at 3 weeks of age or older is possible in the presence of maternal immunity,5 it is not known if immunity is as effective or persists as long in these pigs as it does in pigs vaccinated in the absence of maternal immunity. It seems prudent to delay vaccination until maternal immunity has waned if possible.
'Default' vaccination timing
While seroprofiling (ie, determining the serologic status of various age groups of pigs to assess infection timing and incidence) is the recommended method of identifying the optimal time to vaccinate, seroprofiling results and vaccination responses of numerous herds have revealed that transmission of L intracellularis infection often begins in the nursery rather than later, in the traditional finishing phase of production (10 to 26 weeks of age) (Boehringer Ingelheim Vetmedica, Inc, Technical Services, unpublished data, 2004).6 In the absence of herd-specific diagnostic profiling to accurately identify proper vaccination timing, a recommended 'default' time frame for L intracellularis vaccination in North American pork production systems is the mid-nursery phase (ie, 5 to 8 weeks of age). Adjustments in timing may be required periodically on the basis of observed responses to vaccination and changes in infection dynamics in individual herds.
The immunoperoxidase monolayer antigen assay
Seroprofiling using the immunoperoxidase monolayer antigen (IPMA) assay is an antemortem technique to identify the period when exposure to L intracellularis infection occurs within a pork production system. Lawsonia intracellularis IPMA testing services are available from the University of Minnesota (St Paul, Minnesota) and Boehringer Ingelheim Vetmedica, Inc (Ames, Iowa). The sampling schedule and sample sizes are selected to accurately determine if and when natural exposure begins in a population, so that vaccination may be properly timed to achieve optimal long-term immunologic protection against porcine proliferative enteropathy (PPE, ileitis).
Animals exposed to L intracellularis develop serum IgG antibodies specific for the organism. The reported percentage of agreement between the cell culture indirect fluorescent antibody test (IFAT) and the IPMA is 98.6%.7 However, the IPMA test is easier to perform and interpret. Validation of the IPMA showed a specificity of 100% and a sensitivity of 88.9% in animals experimentally infected with L intracellularis.8
Blood samples should be allowed to clot for several hours at a temperature < 25°C. Serum may be stored at 2 to 8°C for up to 1 week, or in a non-frost-free freezer at -20°C or lower for longer storage times. Submit 2 mL of serum (minimum required, approximately 0.5 mL). Sera are tested at a 1:30 dilution. Lower dilutions are not used, as positive results are indistinguishable from nonspecific binding.
For interpretation, test wells are compared to positive and negative control sera. Wells are considered positive if reddish vibrioid bacterial forms are visible both within and around the infected cells. Positive results indicate that the animal has IgG antibodies to L intracellularis, induced by either a recent or earlier infection. Negative results indicate that the animal has either not been exposed to L intracellularis, has been exposed within 2 weeks prior to sample collection, or has been exposed at some time, but antibody levels have fallen below the limit of detection. It is important to note that pigs that have been exposed to natural infection in the presence of maternal immunity or antibiotics effective against L intracellularis may not seroconvert as expected,9 and this possibility should be considered when serological results are interpreted. Recording the feed medication program in use when the animals are sampled facilitates interpretation.
ELISA tests have recently been developed10,11 and, pending validation, may become commercially available. ELISA tests would presumably offer faster turn-around time and potentially be less expensive than IPMA and IFA tests.
Seroprofiling sampling strategy
Blood samples should be collected at 4-week to 6-week intervals, since anti- L intracellularis serum IgG antibodies may be short-lived (3 to 6 weeks) in response to mild to moderate disease, eg, porcine intestinal adenomatosis (PIA).4,6,12 However, antibody levels may persist for significantly longer periods of time (up to 3 months) following severe, acute L intracellularis infection causing proliferative hemorrhagic enteropathy (PHE).4 An example of a sampling schedule is shown in Figure 2.

Sample size selection should place emphasis on providing a reasonably high level of confidence that a low prevalence of infection (eg, 5%) will be detected early. It is commonly recommended that 30 samples be collected per age group, providing 80% confidence that early infection with a low infection prevalence will be detected. Collection of only 10 samples per age group provides only 40% confidence that a low infection prevalence will be detected. Tables 1 and 2 provide an overview of the relationships between infection prevalence, sample size, and confidence levels of detecting infection.
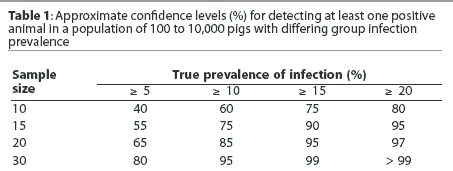
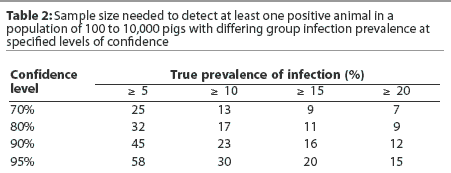
Additional sampling considerations
If diagnostic confirmation of L intracellularis infection in a herd occurs earlier than any of the recommended sampling times, it is not necessary to sample the herd at the later times.
It is important to note that even though only 4% of US farrow-to-finish herds were seronegative for L intracellularis in a 1995 national serologic survey,13 only 37% of producers actually recognized ileitis as a problem in their herds in a subsequent national survey,14 suggesting that subclinical infection is common. Results of a Canadian investigation imply that 40% of Ontario herds are subclinically infected,15 ie, no diarrhea or other symptoms are readily apparent, even though PPE lesions are present and impairing productivity. Within herds, subclinical infection is reported in 40%16 to 50%17 of pigs.
For several reasons, the onset of clinical signs is less reliable as an indicator for estimating the time of infection than is seroconversion. First, many pigs are subclinically infected. Second, clinical signs are not specific to L intracellularis infection, and fecal polymerase chain reaction (PCR) must be performed on several diarrheic fecal samples to determine whether L intracellularis is being shed. While the fecal PCR test is highly specific, it has low sensitivity, resulting in a relatively high false-negative rate.18 However, if PCR-positive fecal samples are identified, it is likely that L intracellularis is contributing to the observed diarrhea. Third, the onset of clinical signs may occur several weeks after the onset of seroconversion in cases of natural exposure (T. Gillespie, personal communication, 2004), and the observation of clinical signs is therefore less reliable than seroconversion as a predictor of onset of infection. Immunohistochemistry on fixed sections of intestinal tract is currently the gold standard for confirming L intracellularis infection, but is, of course, a postmortem test.
Antibiotic medication may 'mask' the serologic response to L intracellularis exposure,19,20 and changes in feed or water antibiotic regimens may change the dynamics of infection. Therefore, we recommend that any contemplated changes in feed medication programs be made prior to seroprofiling, and that blood samples be drawn from pigs on the new regimen so that results are representative of current infection dynamics.
Interpretation
Application of the serologic test should be limited to confirming that clinical or subclinical L intracellularis infection is present and identifying the age of onset of infection so that intervention measures may be properly timed. A positive serologic test in response to active immunity is indicative of exposure to L intracellularis infection, but does not confirm that lesions are present or that observed clinical signs are due to PPE. Positive serologic test results believed to be due to maternally derived antibodies have been observed in pigs up to at least 5 weeks of age (unpublished data, Boehringer Ingelheim Vetmedica, Inc, Technical Services Department).4 Kroll et al5 have shown that recently weaned pigs can be actively immunized in the presence of maternally derived serum antibodies; however, it is unknown whether this protection is as strong or lasts as long as in pigs vaccinated when they have no detectable maternal antibody. It is not known if suckling piglets can be actively immunized in the presence of maternal immunity. A small study conducted in Australia9 suggests that piglets suckling immune sows may be resistant to infection. If confirmed, this information implies that piglets suckling immune sows should not be vaccinated.
The serologic response to L intracellularis vaccination is inconsistent, ranging from 0% of pigs in some studies (unpublished data, Boehringer Ingelheim Research and Development Department)21 to 100% in another study;22 therefore, serology should not be considered a valid tool for monitoring vaccination compliance. Currently available serologic tests cannot differentiate between seroconversion in response to vaccination and seroconversion in response to natural infection. Vaccination does not prevent seroconversion due to subsequent natural exposure. In fact, seroprofiles of vaccinated and nonvaccinated groups of pigs naturally exposed to L intracellularis appear to be similar (S. Dritz and D. Walter, personal communication, 2004). Since serum IgG is not considered to be a major protective factor in diseases limited to the enteric tract, it is not surprising that seronegative vaccinates have repeatedly been protected in challenge studies (unpublished data, Boehringer Ingelheim, Inc, Research and Development Department).1,23 Cellular immunity has been induced both by challenge infection and vaccination and is likely to be an important protective factor,22 as is mucosal immunity (secretory IgA), also sometimes referred to as "local" immunity.24
Reduced dosing of vaccine
Reduced dosing of vaccines (off-label use of a dose lower than recommended by the manufacturer and approved by regulatory authorities) is sometimes practiced in an attempt to reduce input costs.25 Vaccine manufacturers are required by US federal regulatory authorities to quantify the effective dose of a vaccine as a prior condition of licensure. This defined dose is then used to profile onset of immunity, duration of immunity, maternal interference, and other relevant vaccine performance characteristics. Using a reduced dose threatens to compromise all of these benefits. Manufacturer studies are performed under controlled conditions26 so that the dose response can be accurately and validly characterized.27 Attempts to perform dose-determination 'trials' in the field may be confounded by both recognized and unrecognized factors, as commercial farms and pig production processes are not designed to accommodate the level of control and precision needed to accurately measure the required parameters. We strongly discourage such practices and recommend adhering to animal health product manufacturers' scientifically proven and legally approved directions for product use.
Summary
Proper use of available antemortem serologic tests can confirm the need, and allow more precise scheduling, for L intracellularis vaccination. Vaccination should be scheduled for a time at least 8 weeks before observed seroconversion due to natural exposure. Vaccination is often more appropriately scheduled in the nursery phase of production (eg, 5 to 8 weeks of age) rather than in the finishing phase. Lawsonia intracellularis serology cannot reliably be used as a tool for monitoring vaccination compliance or to differentiate seroconversion due to field infection or vaccination. Patterns of antibiotic usage in seroprofiled populations should be recorded and taken into account when results are interpreted, because administration of antibiotics may inhibit L intracellularis seroconversion.
References
1. Kroll JJ, Roof MB, McOrist S. Evaluation of protective immunity in pigs following oral administration of an avirulent live vaccine of Lawsonia intracellularis. Am J Vet Res. 2004;65:559-565.
*2. Kolb J, Sick F, Walter D. Efficacy of an avirulent live Lawsonia intracellularis vaccine in pigs. Proc IPVS. Hamburg, Germany. 2004:437.
*3. Waddell J, Sherlock P, Walter D, Kroll J. Ileitis vaccination controls ileitis in the complete absence of oral antibiotics in a start-up breeding farm. Proc AASV. Orlando, Florida. 2003:245-246.
4. Guedes RMC, Gebhart CJ, Armbruster GA, Roggow BD. Serologic follow-up of a repopulated swine herd after an outbreak of proliferative hemorrhagic enteropathy. Can J Vet Res. 2002;66:258-263.
*5. Kroll J, Roof M, Elbers K, Utley P. Maternal immunity associated with Lawsonia intracellularis exposure and vaccination. Proc IPVS. Hamburg, Germany. 2004:255.
6. Just SD, Thoen CO, Thacker BJ, Thompson JU. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commercial swine production system. J Swine Health Prod. 2001;9:57-61.
7. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA. A comparative study of an indirect fluorescent antibody test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:420-423.
8. Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:528-530.
*9. Collins AM, van Dijk M, Vu NQ, Pozo J, Love RJ. Immunity to Lawsonia intracellularis. Proc Allen D Leman Swine Conf. St Paul, Minnesota. 2001;28:115-120.
*10. Keller C, Ohlinger VF, Kump A, Gratz T, Grunert H, Sieverding E, Heggemann R, Hobfeld P. Herd profiles of antibodies against Lawsonia intracellularis in German farms using a new blocking ELISA. Proc IPVS. Hamburg, Germany. 2004:253.
*11. Boesen HT, Jensen TK, Moller K, Jungersen G. Evaluation of an enzyme-linked immunosorbent assay (ELISA) as a serologic test for Lawsonia intracellularis the agent of proliferative enteropathy. Proc IPVS. Hamburg, Germany. 2004:251.
12. Lawson GH, Gebhart CJ. Proliferative enteropathy: a review. J Comp Pathol. 2000;122:77-100.
13. Bronsvoort M, Norby B, Bane DP, Gardner IA. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J Swine Health Prod. 2001;9:285-290.
14. NAHMS Swine 2000 Part II: Reference of Swine Health and Health Management in the United States, 2000. www.aphis.usda.gov/vs/ceah/cnah/swine/swine.htm. (Resource appears to have relocated to http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/swine/swine.htm as of October 2004. A PDF is available from that page.)
15. Wilson JB, Pauling GE, McEwen BJ, Smart N, Carman PS, Dick CP. A descriptive study of the frequency and characteristics of proliferative enteropathy in swine in Ontario by analyzing routine animal health surveillance data. Can Vet J. 1999;40:713-717.
16. Jacobson M, Hard af Segerstad C, Gunnarsson A, Felsstrom C, de Verdier Klingenberg K, Wallgren P, Jensen-Waern M. Diarrhoea in the growing pig - a comparison of clinical, morphological, and microbial findings from animals between good and poor performance herds. Res Vet Sci. 2003;74:163-169.
*17. Pohlenz J, Meyer C, Schmidt U, Keller C, Ohlinger V. Lawsonia intracellularis: Do subclinical infections exceed clinical cases in relevance for pig production? Proc Conf Res Workers Anim Dis. Chicago, Illinois. 2003. Abstract 94.
18. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am J Vet Res. 1998;59:722-726.
19. Schwartz K, Knittel J, Walter D, Roof M, Anderson M. Effect of oral tiamulin on the development of porcine proliferative enteropathy in a pure-culture challenge model. J Swine Health Prod. 1999;7:5-11.
20. Walter D, Knittel J, Schwartz K, Kroll J, Roof M. Treatment and control of porcine proliferative enteropathy using different tiamulin delivery methods. J Swine Health Prod. 2001;9:109-115.
*21. Connor J, Winkelman N, Gebhart C, Deen J, Wolff T. Inclusion of BMD or BMD plus 3-nitro in swine diets during ileitis vaccination. Proc AASV. Des Moines, Iowa. 2004:131-134.
22. Guedes RMC, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135-145.
*23. Roof MB. Vaccinating for ileitis. Proc Allen D Leman Swine Conf. St Paul, Minnesota. 2001:121-126.
24. Guedes R. Porcine Proliferative Enteropathy: Diagnosis, Immune Response and Pathogenesis [PhD thesis]. St Paul, Minnesota: University of Minnesota; 2002.
*25. Loula TJ. Ileitis: Current thoughts on prevention. Proc AASV. Des Moines, Iowa. 2004:511-513.
*26. USDA-APHIS-Veterinary Services Memorandum No. 800.301. Good Clinical Practice. 2001. www.aphis.usda.gov/vs/cvb/memos/memo800_301.pdf.
*27. Walter D, Kroll J, Holck JT, Okkinga K. Observations of dose dependency with Enterisol" Ileitis vaccine. Proc AASV. Des Moines, Iowa. 2004:261-262.
* Non-refereed references
